FDA has released its briefing document ahead of their Dec 10 meeting to request emergency use licensing:
Here is the data submitted by Pfizer (92 pages): https://www.fda.gov/media/144246/download
Here is the FDA briefing review (53 pages):
https://www.fda.gov/media/144245/download
Here are important takeaways
Here is the data submitted by Pfizer (92 pages): https://www.fda.gov/media/144246/download
Here is the FDA briefing review (53 pages):
https://www.fda.gov/media/144245/download
Here are important takeaways

1st: My expertise alone wasn’t enough to analyze the entire dataset, so I sought input from those far more qualified than myself & I’m grateful for their time & input
2nd: In-depth analysis of the documents will take more time, but I thought I should share some general overview
2nd: In-depth analysis of the documents will take more time, but I thought I should share some general overview
I’m going to write my brief analysis based on the most common questions I’ve received from the public.
So, first let’s tackle the side effects/safety question:
 list of adverse reactions experienced by those vaccinated (% of people experiencing).
list of adverse reactions experienced by those vaccinated (% of people experiencing).
So, first let’s tackle the side effects/safety question:
 list of adverse reactions experienced by those vaccinated (% of people experiencing).
list of adverse reactions experienced by those vaccinated (% of people experiencing).
 injection site reactions (84.1%)
injection site reactions (84.1%) fatigue (62.9%)
fatigue (62.9%) headache (55.1%)
headache (55.1%) muscle pain (38.3%)
muscle pain (38.3%) chills (31.9%)
chills (31.9%) joint pain (23.6%)
joint pain (23.6%) fever (14.2%)
fever (14.2%)
 These’re typical of reactogenicity & are seen in other vaccines too
These’re typical of reactogenicity & are seen in other vaccines too They’re generally mild to moderate & lasted a few days
They’re generally mild to moderate & lasted a few days  They’re more frequent after 2nd dose than 1st
They’re more frequent after 2nd dose than 1st They were less frequent in >55 years of age (<2.8% in older adults vs. ≤4.6% in younger participants)
They were less frequent in >55 years of age (<2.8% in older adults vs. ≤4.6% in younger participants)
There are 2 non-serious side effects reported, but their occurrence did NOT exceed that of what is found in general public.
These are: lymphadenopathy (swollen lymph nodes) & Bell’s palsy. They were both temporary and resolved with time.
These are: lymphadenopathy (swollen lymph nodes) & Bell’s palsy. They were both temporary and resolved with time.
Of course participants are being monitored for long-term effects, but so far FDA reviewers saw no cause for serious safety concern!
 Not enough data for <16 yrs old, pregnant/breastfeeding women, immunocompromised (no signs of disease enhancement with current data available)
Not enough data for <16 yrs old, pregnant/breastfeeding women, immunocompromised (no signs of disease enhancement with current data available)
 Not enough data for <16 yrs old, pregnant/breastfeeding women, immunocompromised (no signs of disease enhancement with current data available)
Not enough data for <16 yrs old, pregnant/breastfeeding women, immunocompromised (no signs of disease enhancement with current data available)
For efficacy, not much has changed since their press release ( is my Nov 9
is my Nov 9  on it), though they’ve recruited 5k more participants up to Nov 14.
on it), though they’ve recruited 5k more participants up to Nov 14.
 Majority of efficacy data is for >16yrs, w/ a few data points available on 12-16 yrs.
Majority of efficacy data is for >16yrs, w/ a few data points available on 12-16 yrs.
 ~21.5% of participants are >65 yrs https://twitter.com/denovo_fatima/status/1326002593194184707
~21.5% of participants are >65 yrs https://twitter.com/denovo_fatima/status/1326002593194184707
 is my Nov 9
is my Nov 9  on it), though they’ve recruited 5k more participants up to Nov 14.
on it), though they’ve recruited 5k more participants up to Nov 14. Majority of efficacy data is for >16yrs, w/ a few data points available on 12-16 yrs.
Majority of efficacy data is for >16yrs, w/ a few data points available on 12-16 yrs. ~21.5% of participants are >65 yrs https://twitter.com/denovo_fatima/status/1326002593194184707
~21.5% of participants are >65 yrs https://twitter.com/denovo_fatima/status/1326002593194184707
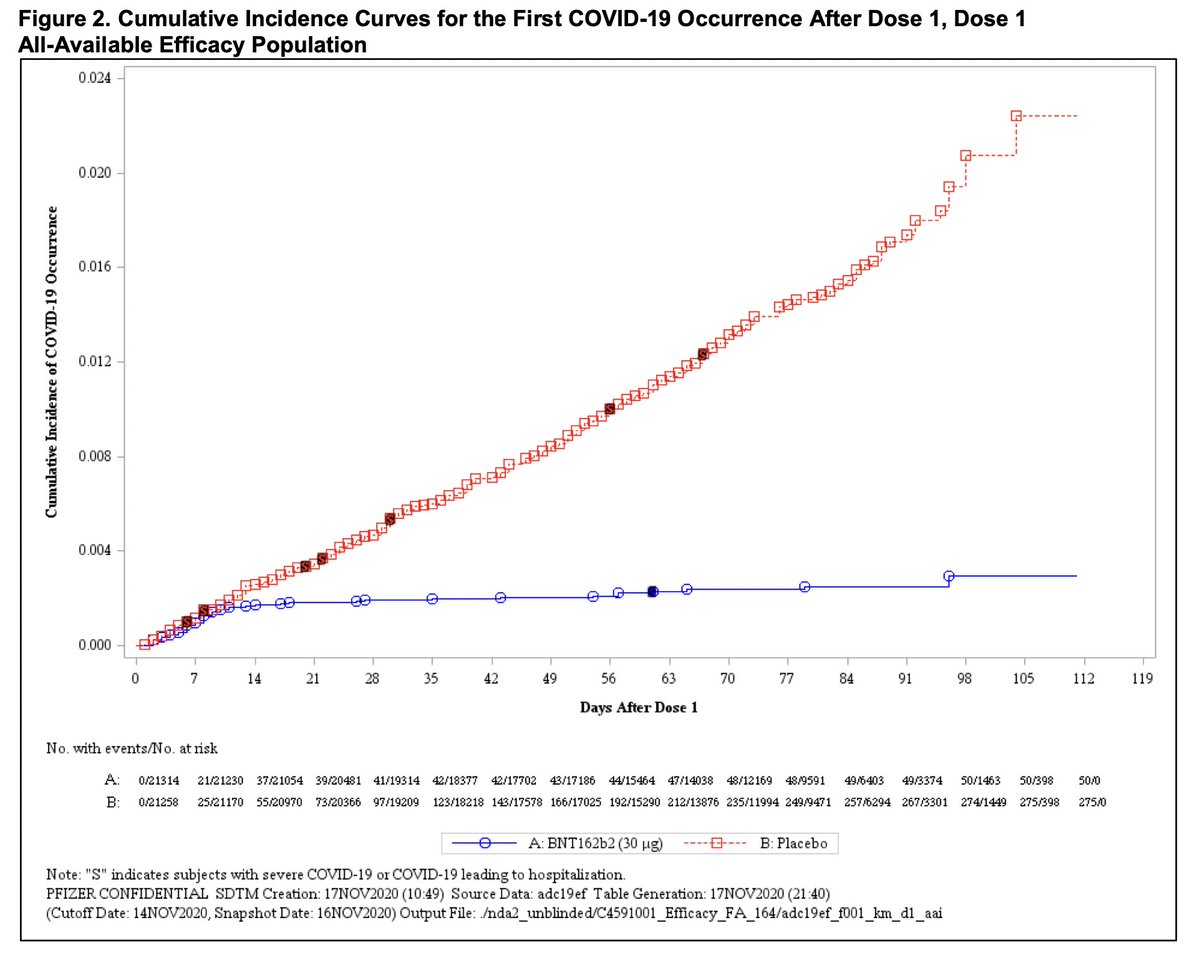
This graph captures REALLY WELL what vaccines do, the red dots are incidents of COVID in those who received placebo, vs. those who received the vaccine (blue).
I hope the people who volunteered for this trial, knowing they might get placebo are prioritize for the vaccine!
I hope the people who volunteered for this trial, knowing they might get placebo are prioritize for the vaccine!
So overall, it’s looking really good actually! I’m a cancer survivor (two different cancers one at a very young age, & one a few years ago) and I feel comfortable receiving this vaccine.
#VaccinesWork
#VaccinesWork
I’d like to address 2 most common questions I get about #COVID vaccines:
 Have they tested them on enough people?
Have they tested them on enough people?
 Does the speed of development mean something sinister is going on?
Does the speed of development mean something sinister is going on?
 Have they tested them on enough people?
Have they tested them on enough people? Does the speed of development mean something sinister is going on?
Does the speed of development mean something sinister is going on?
Answer to 
Effectiveness of a study is determined by its power: the number of “events” that occur during the study (in this case the number of people who got sick w/ Covid.) So having 100K or 1mil isn’t as meaningful as having enough events that empower us to calculate efficacy

Effectiveness of a study is determined by its power: the number of “events” that occur during the study (in this case the number of people who got sick w/ Covid.) So having 100K or 1mil isn’t as meaningful as having enough events that empower us to calculate efficacy
Answer to 
The vaccine was developed quickly b/c
 immense tech advances
immense tech advances
 entire
entire  collaborating & allocating resources to its development (wish we’d do the same for other diseases)
collaborating & allocating resources to its development (wish we’d do the same for other diseases)
 extremely
extremely  community transmission = reach required
community transmission = reach required  of events to determine efficacy fast
of events to determine efficacy fast

The vaccine was developed quickly b/c
 immense tech advances
immense tech advances entire
entire  collaborating & allocating resources to its development (wish we’d do the same for other diseases)
collaborating & allocating resources to its development (wish we’d do the same for other diseases) extremely
extremely  community transmission = reach required
community transmission = reach required  of events to determine efficacy fast
of events to determine efficacy fast
No regulatory or safety corners were cut, we’re just fortunate to live in 2020 and not 1918
(I mean forget about a speedy vaccine, can you imagine not having Netflix or Doordash? )
)
(I mean forget about a speedy vaccine, can you imagine not having Netflix or Doordash?
 )
)
 One EXTREMELY important point:
One EXTREMELY important point: It takes our body a bit of
 to build enough antibodies & generate immunity!
to build enough antibodies & generate immunity! from receiving dose 1 till about a week after receiving dose 2 one MUST continue observing
from receiving dose 1 till about a week after receiving dose 2 one MUST continue observing  , #PhysicalDistancing, & other public health measures to avoid infection
, #PhysicalDistancing, & other public health measures to avoid infection
Point of durability of immunity:
This will become clear w/ time. The participants were injected ~ 4 months ago and so that’s however long we know the immunity lasts. These participants are being monitored longterm, so as time goes by we will know more about duration of immunity.
This will become clear w/ time. The participants were injected ~ 4 months ago and so that’s however long we know the immunity lasts. These participants are being monitored longterm, so as time goes by we will know more about duration of immunity.
Efficacy based on ethnicity/comorbidity
Participants come from diverse backgrounds & health conditions; we have enough data to show efficacy is similar in
 Blacks & Latinos/Hispanic
Blacks & Latinos/Hispanic
 People w/ cardiovascular, chronic pulmonary disease, diabetes, hypertension, obesity
People w/ cardiovascular, chronic pulmonary disease, diabetes, hypertension, obesity
Participants come from diverse backgrounds & health conditions; we have enough data to show efficacy is similar in
 Blacks & Latinos/Hispanic
Blacks & Latinos/Hispanic People w/ cardiovascular, chronic pulmonary disease, diabetes, hypertension, obesity
People w/ cardiovascular, chronic pulmonary disease, diabetes, hypertension, obesity

 Read on Twitter
Read on Twitter