With the . @NIAIDNews #ACCT1 trial final report published on 05 Nov 2020 and the . @WHO's #SolidarityTrial Preliminary report published on 02 December 2020, thought it would be good to take another look at both trials and find ways forward for the patients we are dealing with today
Motivated by a patient I am dealing with who will survive very severe #COVID19 in the setting of transplant, but her father, who was hospitalized earlier than her with moderate COVID, was left to progress and died before her discharge, given #remdesivir only after going on #BiPAP
Here is the link to the prior analysis and I think the new publications give some interesting details which will follow https://twitter.com/FranciscoMarty_/status/1318685702003982336?s=20
To some the psychology framework of these #remdesivir trials is one of David vs. Goliath, fighting big pharma greed who snook a useless drug in a time of crisis for profit.
On the other hand, we have a serious team . @US_FDA approving it for regular use: https://www.nejm.org/doi/full/10.1056/NEJMp2032369
On the other hand, we have a serious team . @US_FDA approving it for regular use: https://www.nejm.org/doi/full/10.1056/NEJMp2032369
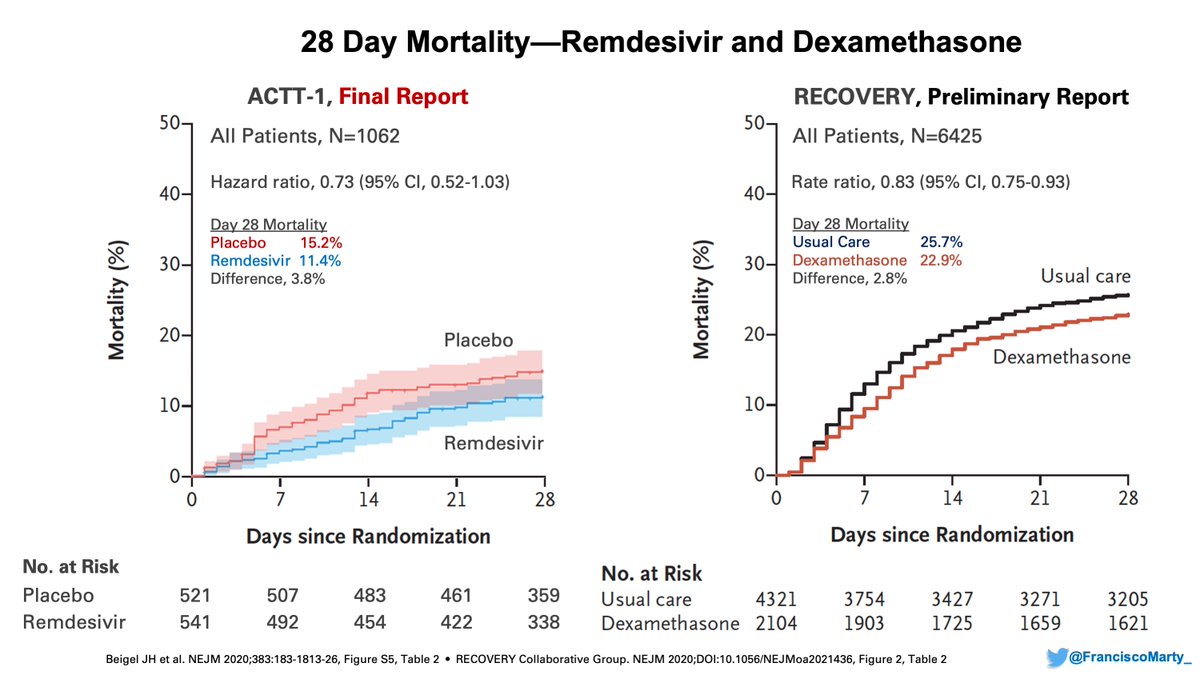
As a quick recap, I wanted to begin by comparing the final results of #ACTT1 to the #RecoveryTrial on #dexamethasone with the updated data.
Both trials show an overall mortality difference of ~3%, one with 1064 patients, not significant, the other with 6425 patients, significant
Both trials show an overall mortality difference of ~3%, one with 1064 patients, not significant, the other with 6425 patients, significant
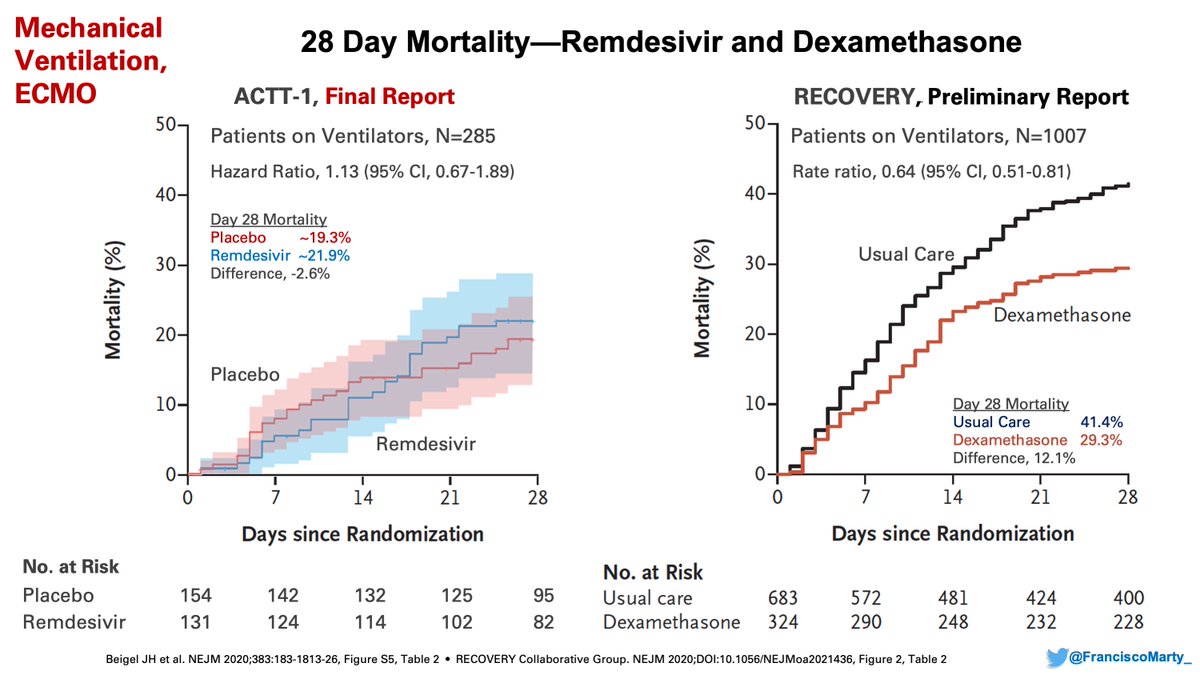
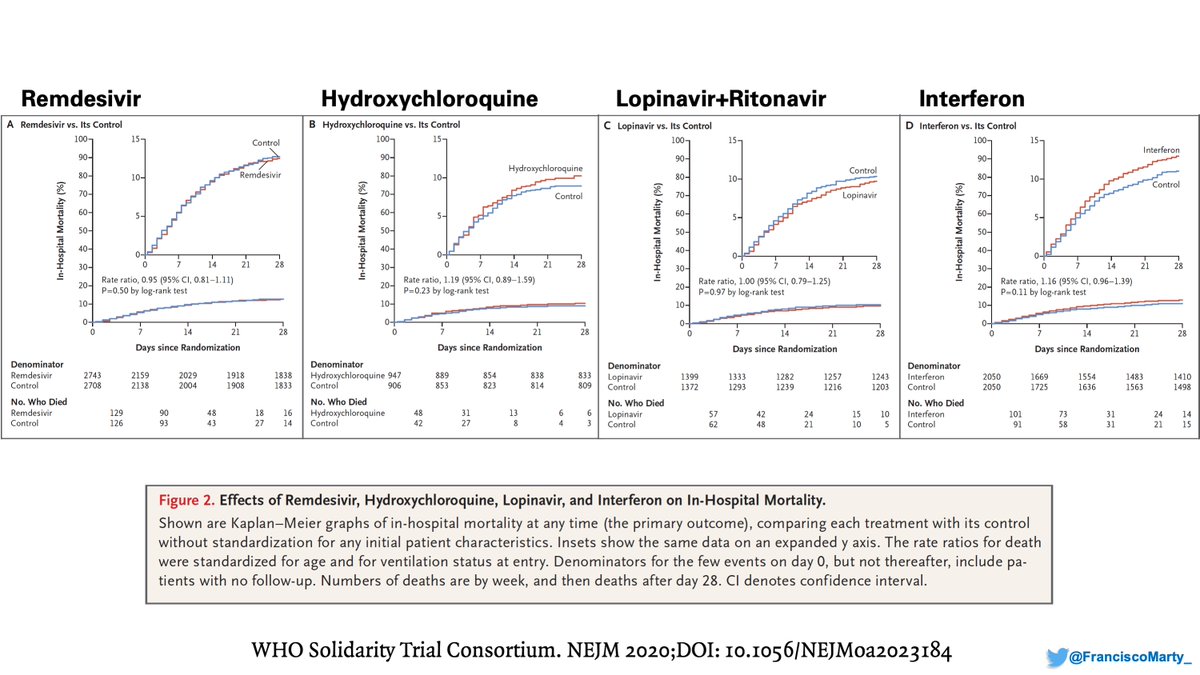
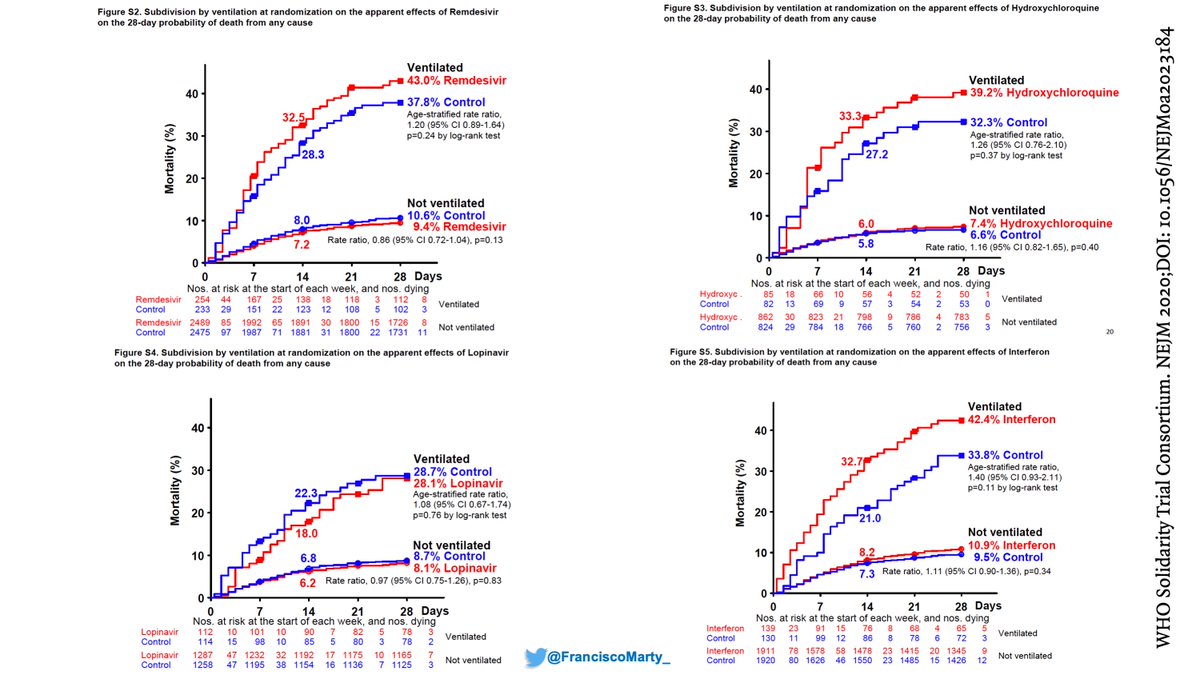
But the benefit was not uniformly distributed. #Remdesivir did not help patients on #ventilators, where as #dexamethasone helped a lot, if anything mortality was numerically higher on remdesivir by day 28 (remember for later)
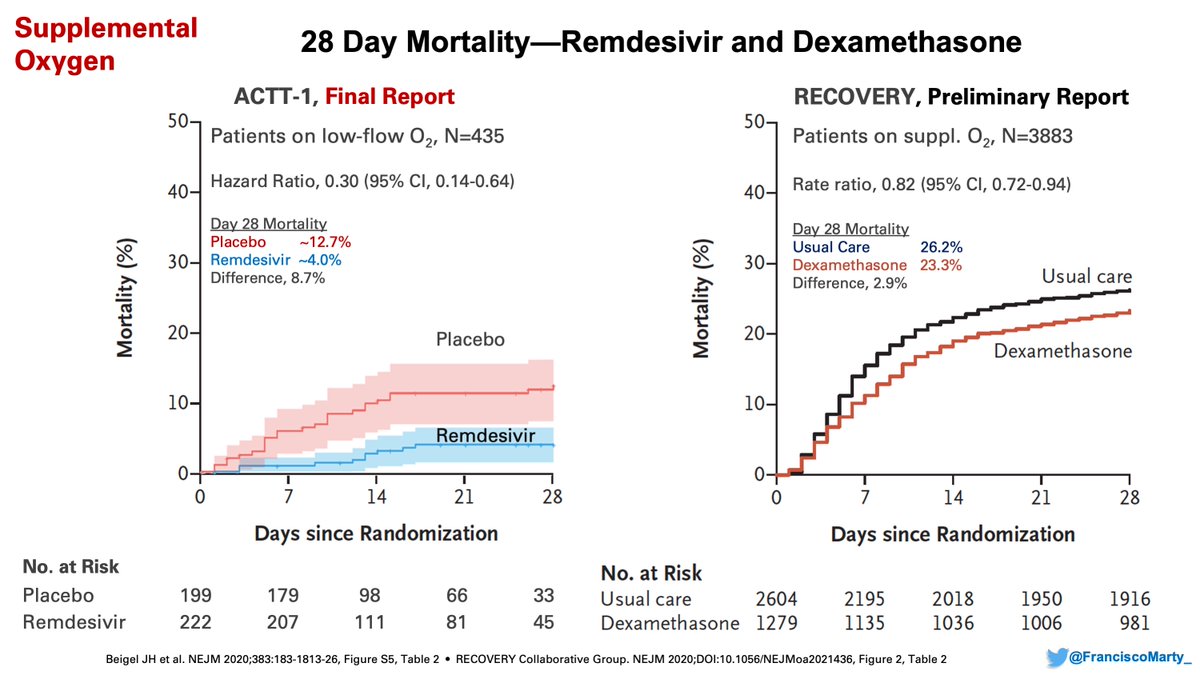
On the other hand, the benefit of #remdesivir was substantial in patients on low-dose oxygen and drove the overall benefit on the trial, whereas #dexamethasone had a modest effect in the setting of a high mortality background of 26%.
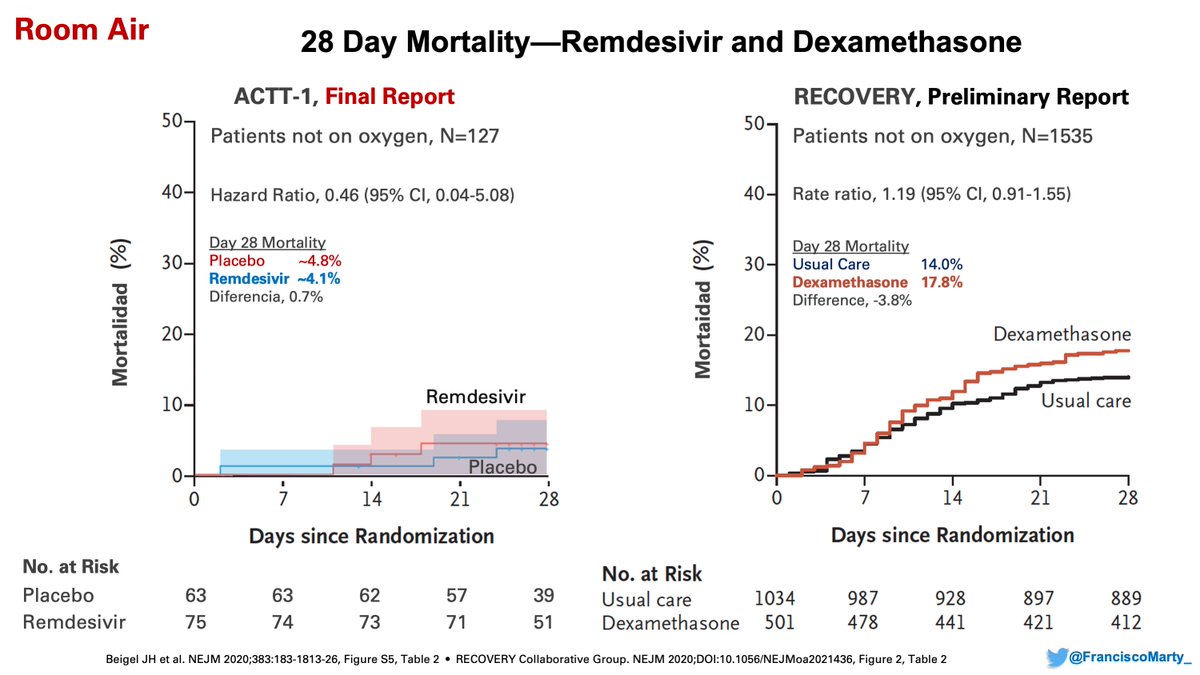
And the caution of potential harm when #dexamethasone may increase mortality when used in patients who do not require oxygen, and a lack of overall effect on mortality for patients not on oxygen (moderate #COVID19)
As many people noticed, there were not many changes from the pre-print to the published . @NEJM version even though, at least for me, there are several key questions the authors did not provide basic information about, and the paper came with its own #metanalysis, also rare
Maybe just my impression, but realized that one of my medical statistical heroes, Sir Richard Peto, was very involved in the design & analysis: he did the statistical work, is part of the steering committee, the writing committee, but (oddly) also the statistician for the DSMB
He basically invented the metanalysis of clinical trials, which may explain why we get one in the paper, he developed and refined the logrank test, and has always been a force behind large simple randomized trials, he came up with the Peto Paradox, to name a few things!
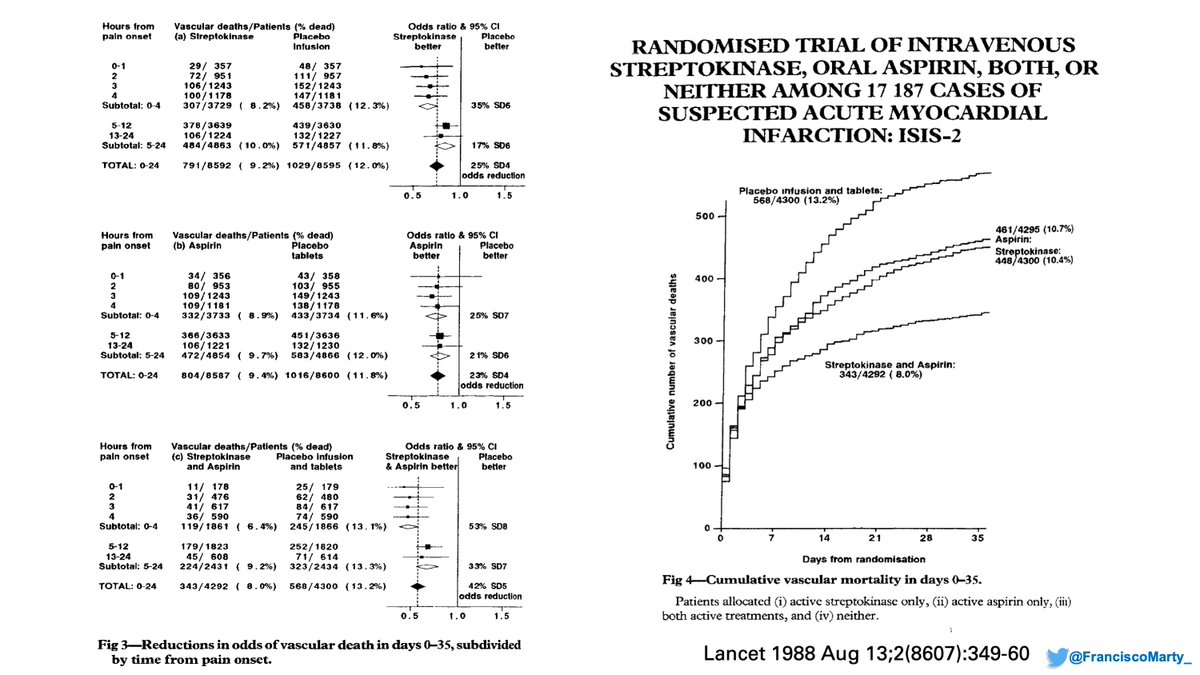
Although much of his career has been devoted to cancer epidemiology, he started and was one of the masterminds behind the classic #ISIS2 trial that compared #streptokinase & repurposed #aspirin for treatment of acute myocardial infarction: https://www.jameslindlibrary.org/wp-data/uploads/2014/07/ISIS-2_Collaborative_Group_1988.pdf
#cardiotwitter
#cardiotwitter
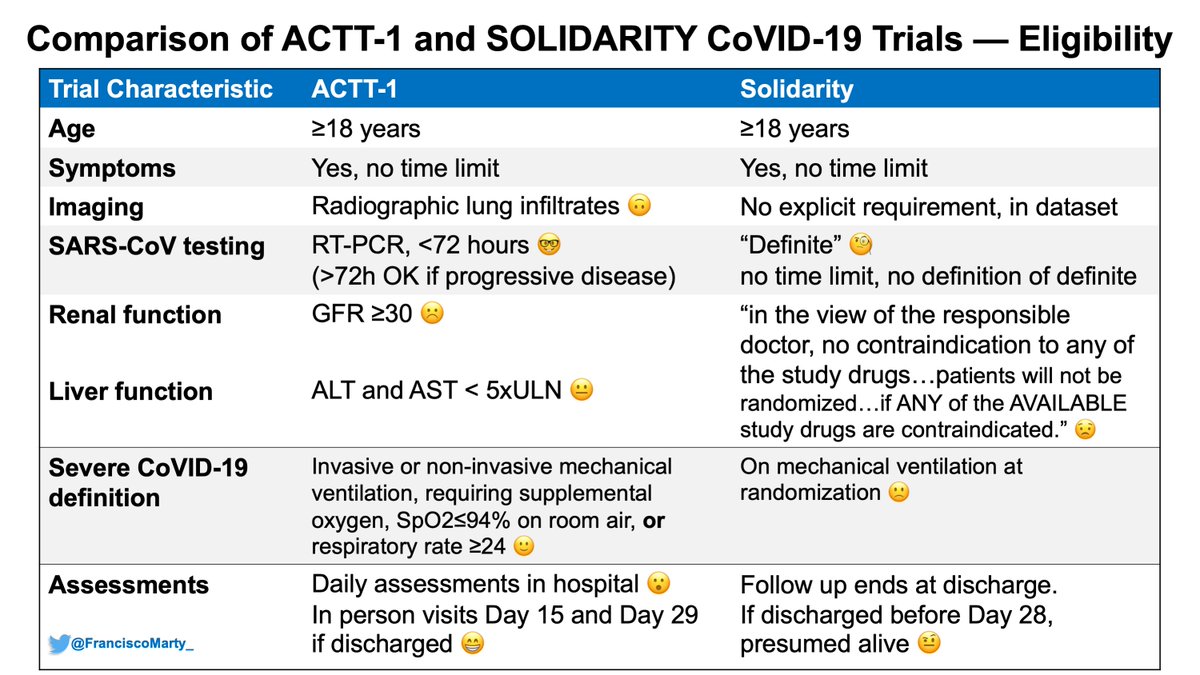
As you read through the #ISIS2 paper (link above) you recognize lots of similar elements & even phrase structures of what you read in the #SolidarityTrial, which I think provides a window to understanding the limitations of the trial, not seen in #ISIS2.
See eligibility below:
See eligibility below:
Timing from onset of symptoms to treatment was important for eligibility, and was collected carefully by the people who received the registration and randomization calls from over 400 sites, and they wanted to make sure doctors were uncertain of the effect of the drugs tested.
That's a huge missing piece from Solidarity, when was onset of #COVID19 symptoms in relation to treatments? Like in cardiology, in ID, time of onset does count as he and the DSMB at the time noted and analyzed for #ISIS2, but this is not presented, or recognized as not collected.
This had been highlighted in previous trials of #remdesivir
-Wang/Wuhan trial, median time from symptom onset to treatment was 10 days; IQR, 9 to 12; only 25% got treated <9 days from onset
-ACTT1, median time 9 days; IQR 6 to 12, 25% got treated ≤6 days from onset.
-Wang/Wuhan trial, median time from symptom onset to treatment was 10 days; IQR, 9 to 12; only 25% got treated <9 days from onset
-ACTT1, median time 9 days; IQR 6 to 12, 25% got treated ≤6 days from onset.
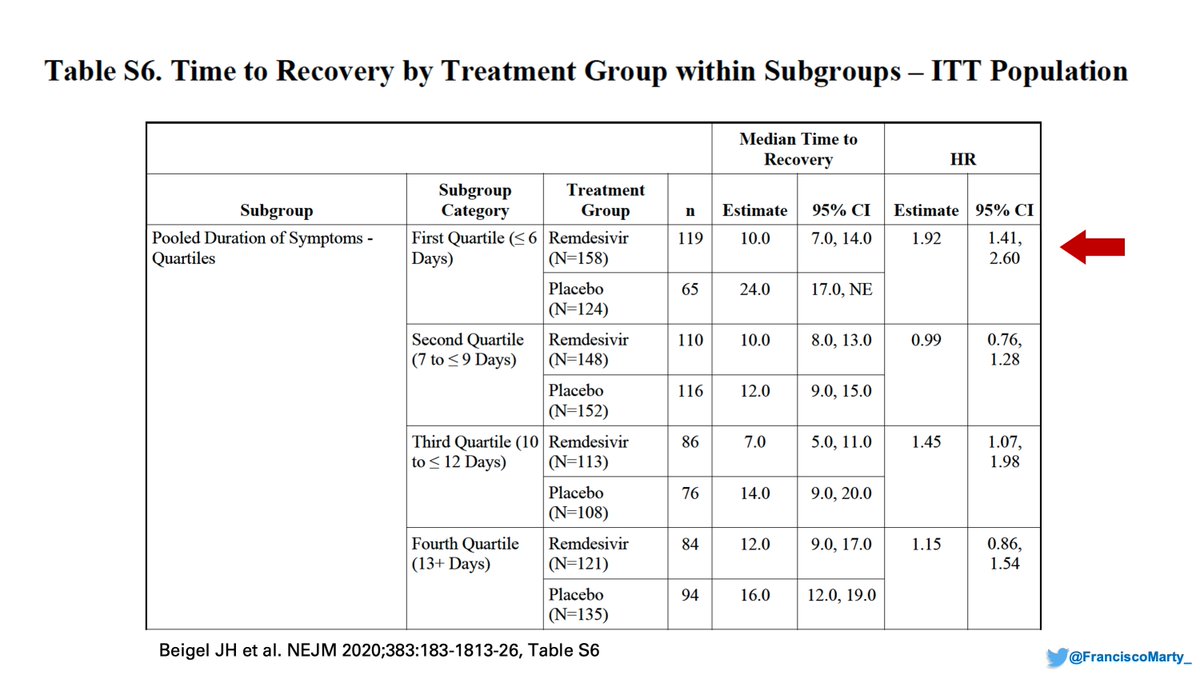
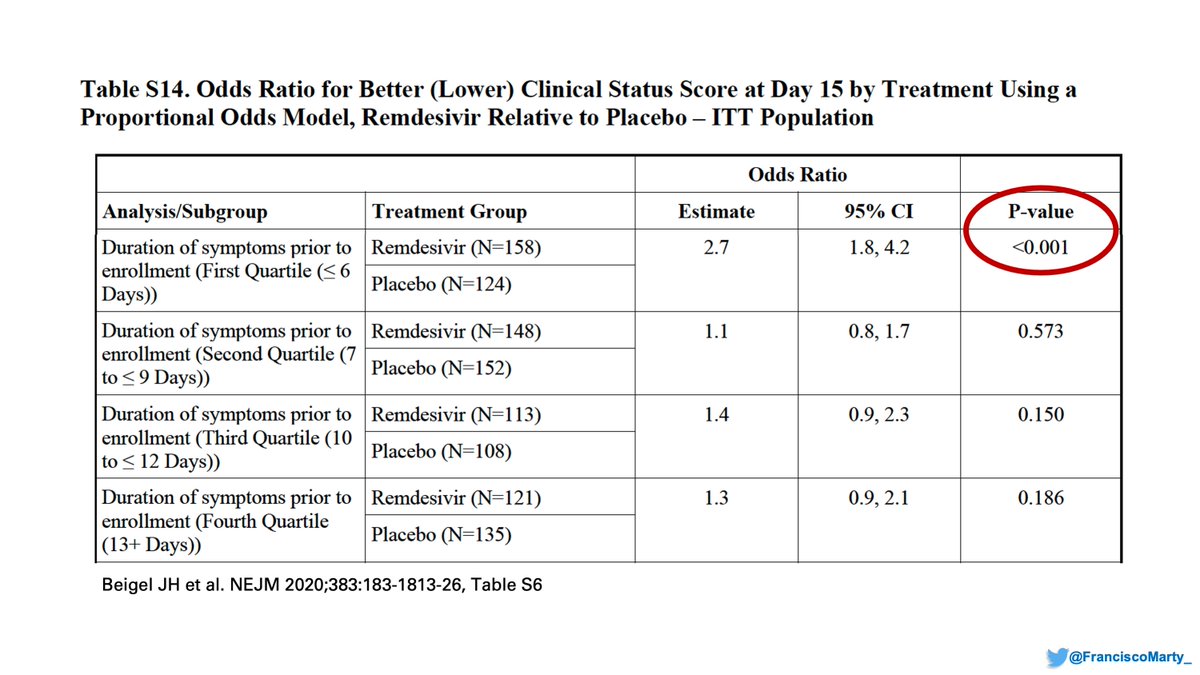
If you look at the final #ACTT1 appendix, you find these cool analyses based on time of onset: people treated in the first quartile of symptoms (≤6 days), did the best in the trial.
I suspect one of the discrepancies between trials is the lack of attention to disease onset.
I suspect one of the discrepancies between trials is the lack of attention to disease onset.
Lets take a look at the rest of the differences between the 2 trials
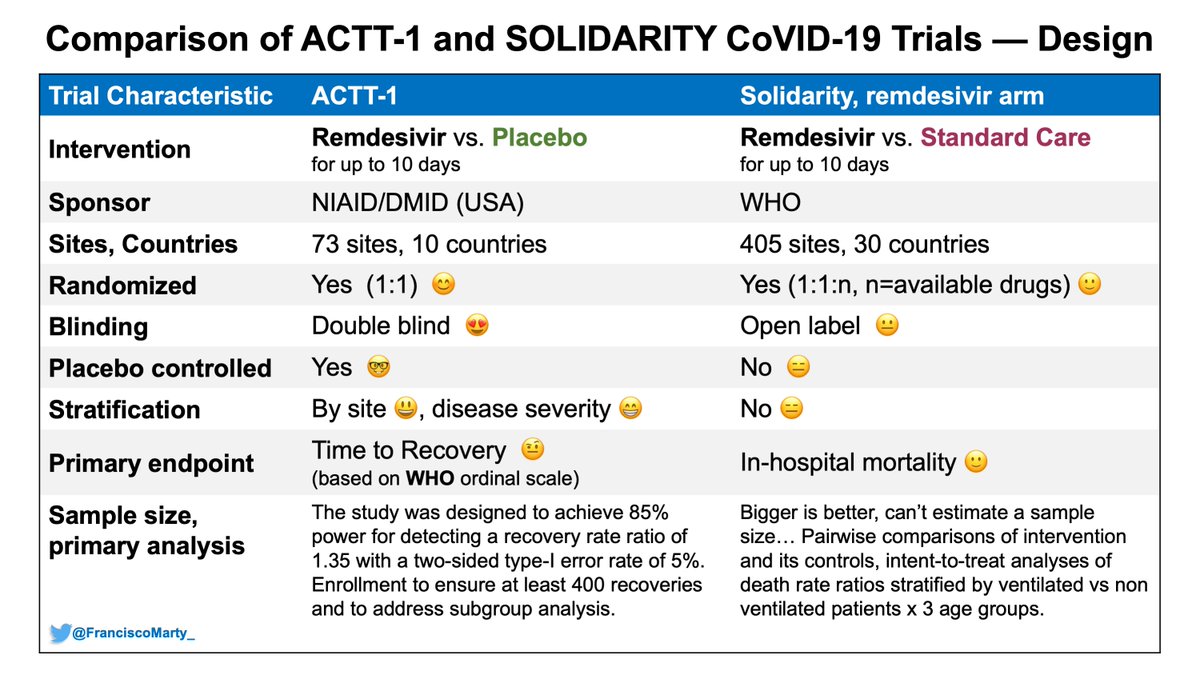
Major issues are the open-label design of Solidarity vs. double-blind, placebo control or #ACTT1 and #ISIS2, and the tricky difference of in-hospital mortality vs all-cause 28 Day mortality.
Major issues are the open-label design of Solidarity vs. double-blind, placebo control or #ACTT1 and #ISIS2, and the tricky difference of in-hospital mortality vs all-cause 28 Day mortality.
Why tricky?
In Solidarity, patients were not followed and presumed alive once discharged after randomization
- The trial database contemplates hospice discharge, not mentioned how many patients went home alive to die at home
- Readmissions, death after readmission not counted
In Solidarity, patients were not followed and presumed alive once discharged after randomization
- The trial database contemplates hospice discharge, not mentioned how many patients went home alive to die at home
- Readmissions, death after readmission not counted
Kind of basic, and likely an inconsequential oversight from the . @NEJM editors, but we are never told how the diagnosis of #SARS-CoV2 and #COVID19 were made, not in the protocol, text, or appendix, just that patients with #definite COVID were enrolled.
This is relevant as the larger the proportion of non-COVID19 COVID look alike, the larger the possibility of bias towards the null. Was 0% in ACTT1, ~10% in Recovery.
The other key crux in the methods, is that it was up to the physician to decide what treatments to randomize to
The other key crux in the methods, is that it was up to the physician to decide what treatments to randomize to
The problem here is that the doctor in front of the screen noted treatments available & contraindicated. The program, according to the protocol, would not allow randomization if any available treatment was contraindicated. The availability was at the patient, not center level.
Why is that relevant?
if a doctor thought a drug was contraindicated, or did not want as an option, would not check it as available for that patient so randomization & allocation would occur, a pre-randomization bias that goes against the clinician's uncertainty key to #ISIS2
if a doctor thought a drug was contraindicated, or did not want as an option, would not check it as available for that patient so randomization & allocation would occur, a pre-randomization bias that goes against the clinician's uncertainty key to #ISIS2
Why I suspect this happened?
ACTT1 results, #remdesivir's EUA, #dexamethasone's Solidarity results were out early in the conduct of Solidarity.
This likely influenced the doctors decision into which arms to make available to each patient, and they did choose differently.
ACTT1 results, #remdesivir's EUA, #dexamethasone's Solidarity results were out early in the conduct of Solidarity.
This likely influenced the doctors decision into which arms to make available to each patient, and they did choose differently.
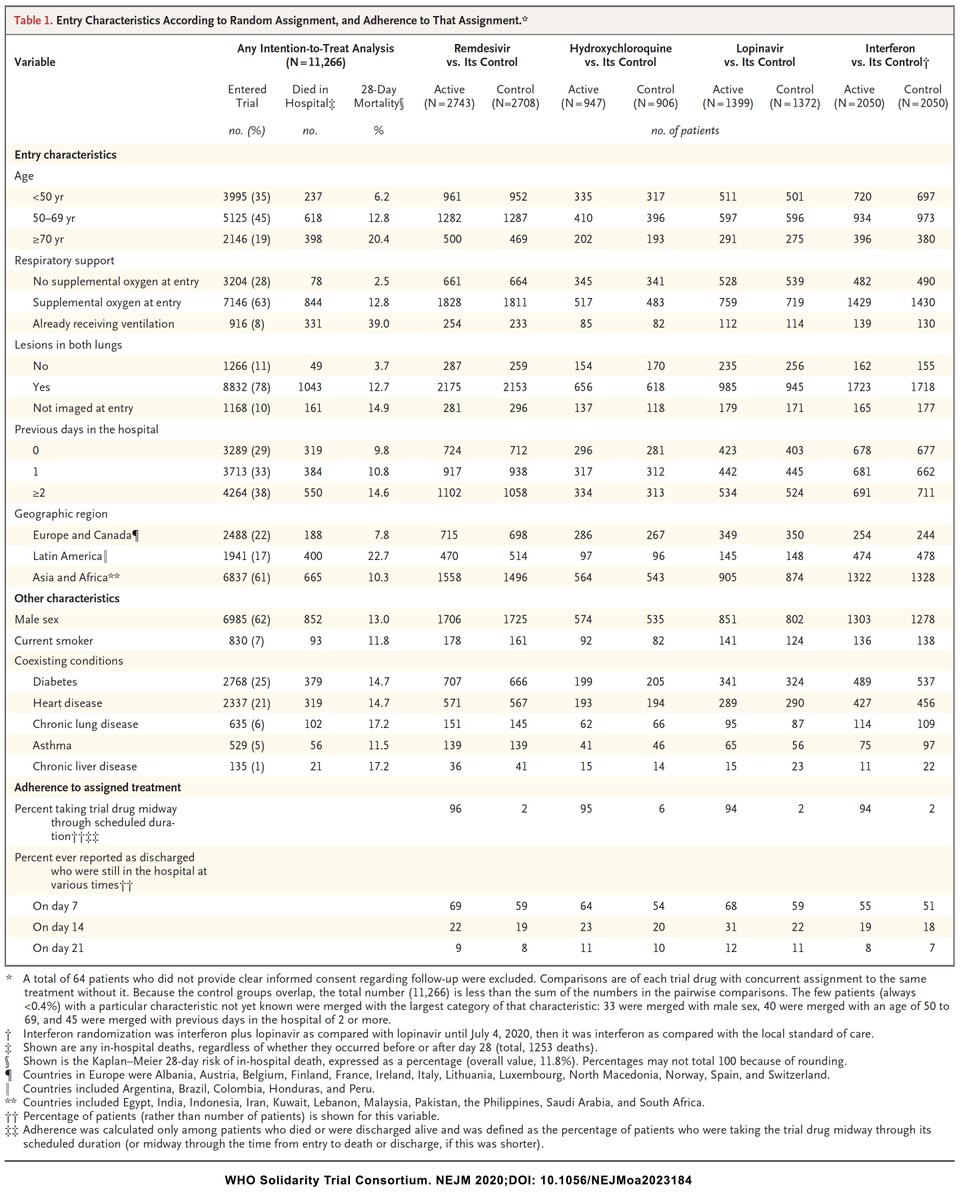
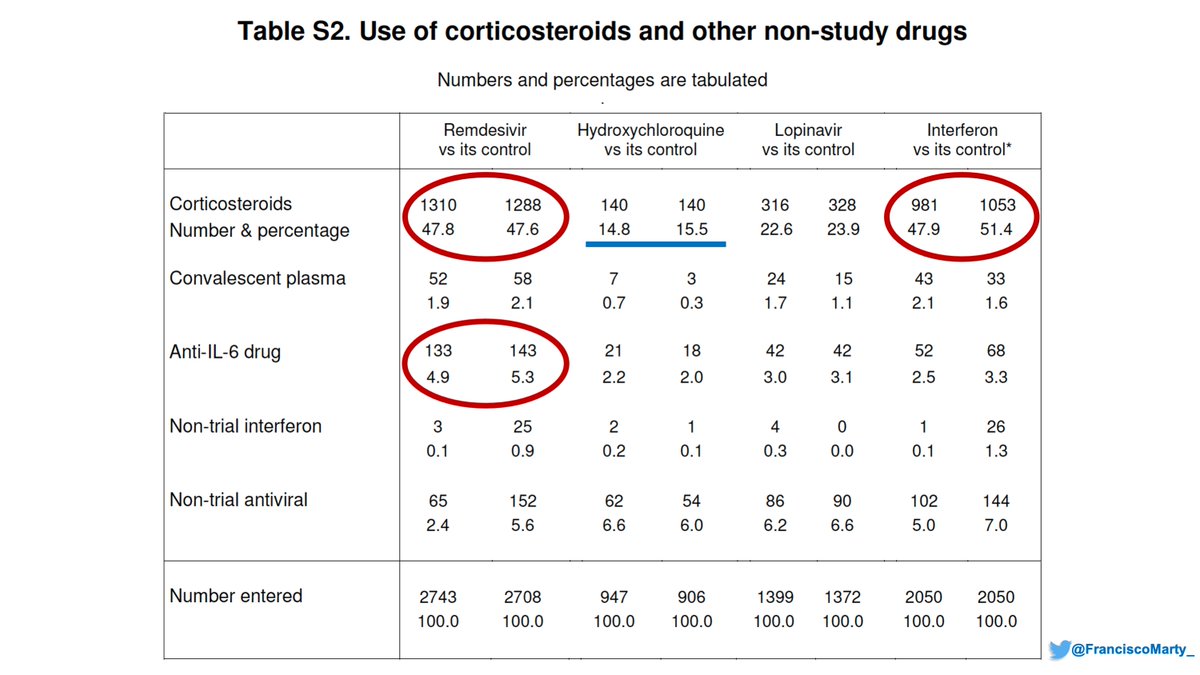
We are not given percentages in Table 1 of the Solidarity paper (merged x u), but if you do the math, you notice that patients who were allocated to #remdsevir or #interferon were sicker than those who were allocated to #HCQ or #lopinavir.
- More need for oxygen, more infiltrates
- More need for oxygen, more infiltrates
As you can see, the background mortality for each arm is different, with almost 4% difference between Remdesivir and HCQ, a huge difference in a trial with thousands of patients
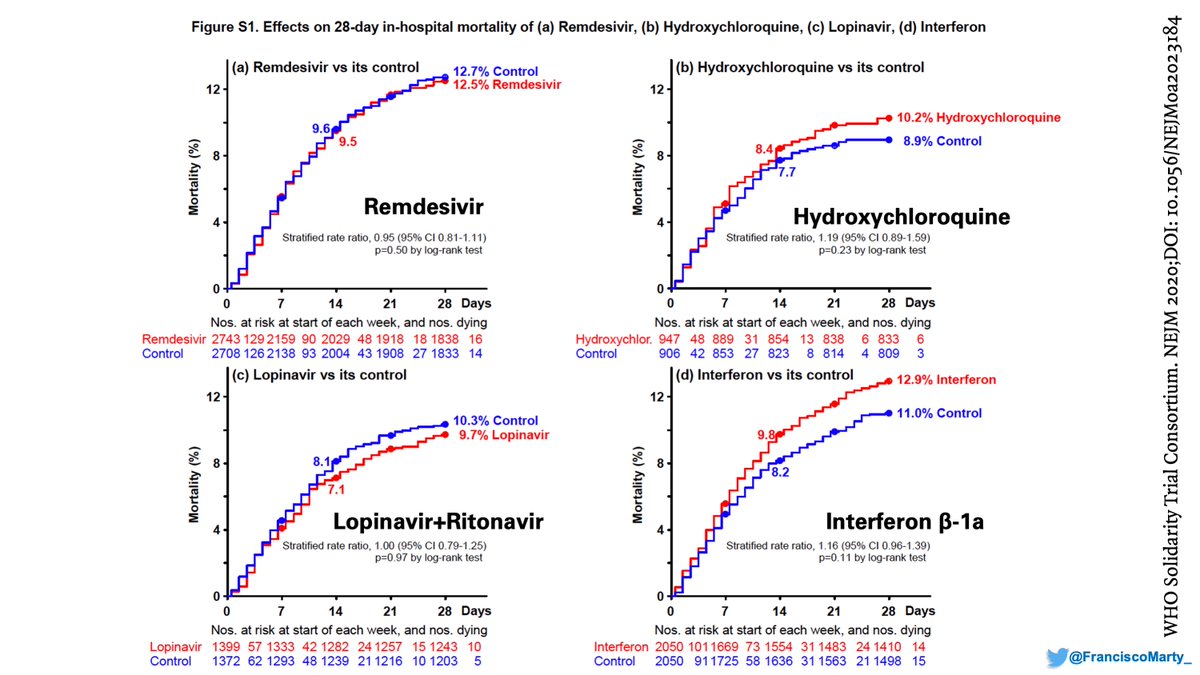
I guess I agree with the writing committee that their own graphs look better and left them in the appendix.
Besides the report on #remdesivir, many people were quite enthusiastic about #interferon, and the data on the #MERS trial was known
Interferon Beta-1b and Lopinavir–Ritonavir for Middle East Respiratory Syndrome https://www.nejm.org/doi/full/10.1056/NEJMoa2015294#.X9AVr_dLUZ8.twitter
Interferon Beta-1b and Lopinavir–Ritonavir for Middle East Respiratory Syndrome https://www.nejm.org/doi/full/10.1056/NEJMoa2015294#.X9AVr_dLUZ8.twitter
Maybe I'm just daydreaming, but then you go to Table S2 of Solidarity Trial and see that almost 50% of patients in the #remdesivir and #interferon arms got #dexamethasone, something everyone started doing in June 2020 for sicker patients compared with 15% of #HCQ
And I think Solidarity suffered from this. If there was certainty some drugs worked better than others the uncertainty to randomize was not there, and like any doctor's reflex, we would offer the sickest the drugs that we had higher certainty of working and contraindicate others
So if the sicker patients, who had a higher probability of presenting later in the course of #COVID19 preferentially were assigned #remdesivir, then the effect of #remdesivir would be diluted, leading to a decreased observed effect.
We fell for this in the Spring when we were running the trials. At the beginning we used to start going for the sickest, those in the ICU, but overtime we realized the best use of #remdesivir was on the wards, preventing progression and transfers to the ICU, we shifted gears.
Then we have the issue of what to do with patients already on mechanical ventilation. Both Solidarity and ACTT suggest not only no benefit, but maybe some harm for these patients that get #remdesivir. Do we have the guidelines upside down?
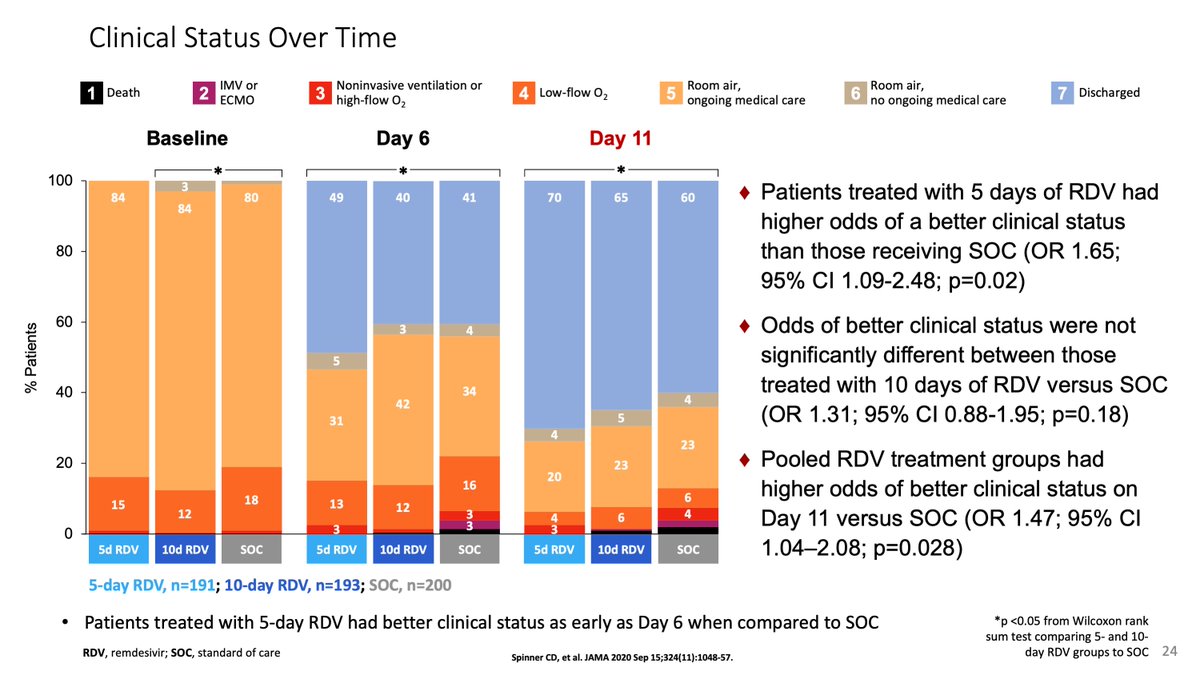
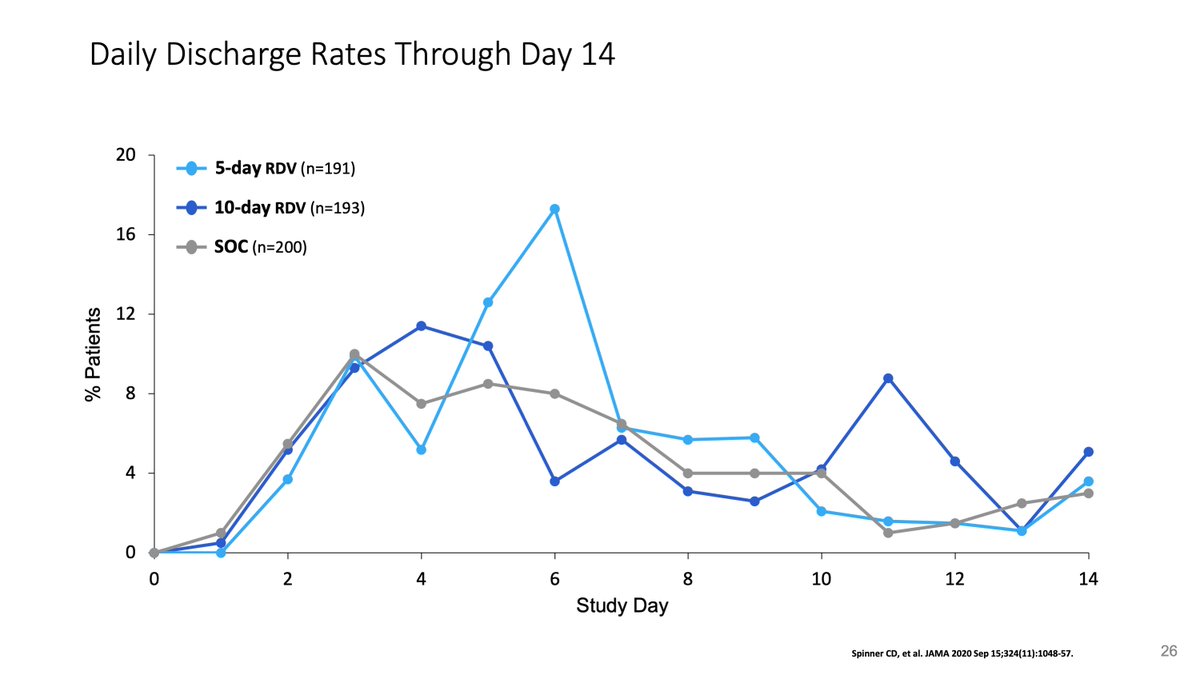
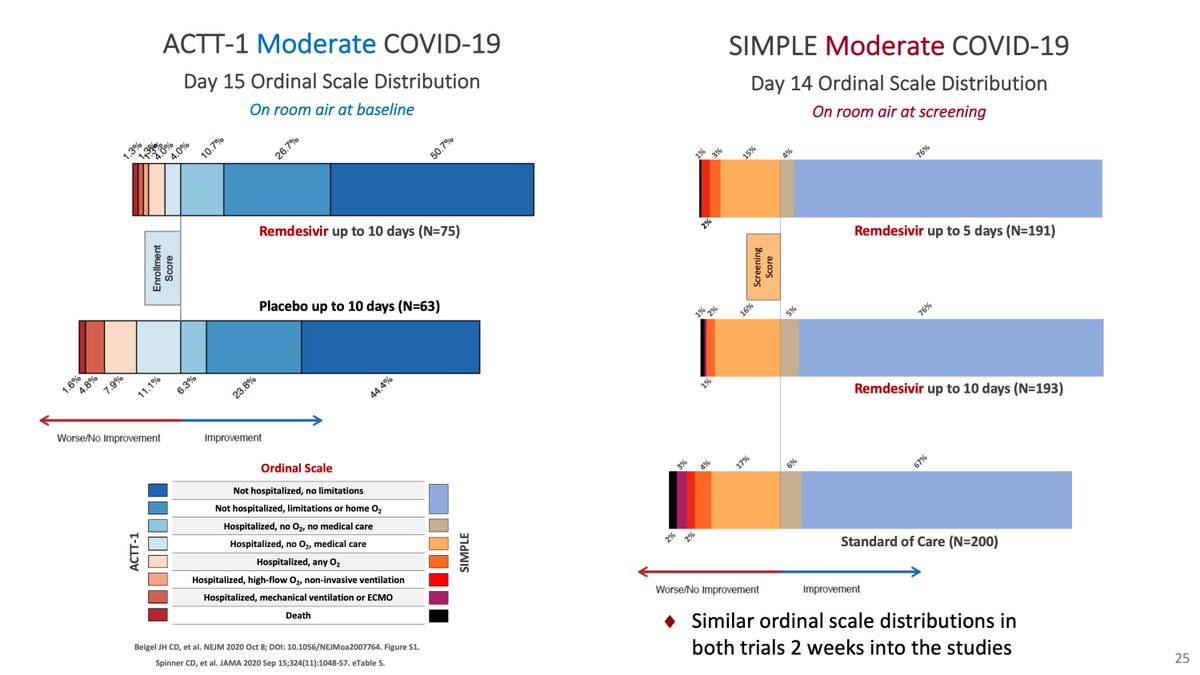
One cool thing about Solidarity is that it confirmed our observation from the SIMPLE moderate that with open-label drug, patients who were getting better, tended to stay in the hospital longer to complete their treatment, 1-3 days longer independent of the drug.
This is key because for the @JAMA_current editors, the lower proportional odds of the Spinner et al paper for those on 10 days of remdesivir meant no meaningful effect, when it was an effect of being open-label.
( https://jamanetwork.com/journals/jama/fullarticle/2769871)
( https://jamanetwork.com/journals/jama/fullarticle/2769871)
The bimodal distribution of doses and discharges were obvious, but we were forced into central tendency measures (medians).
so I think the trials are not contradictory, but reflect different estimates and limitations of #remdesivir use, which like any antiviral should be early in the disease.
After this re-reads, I think the guidelines need to be rethought:
treat all patients with moderate or severe COVID disease with or without oxygen, can't recommend routine use of #remdesivir for patients on mechanical ventilation...
treat all patients with moderate or severe COVID disease with or without oxygen, can't recommend routine use of #remdesivir for patients on mechanical ventilation...
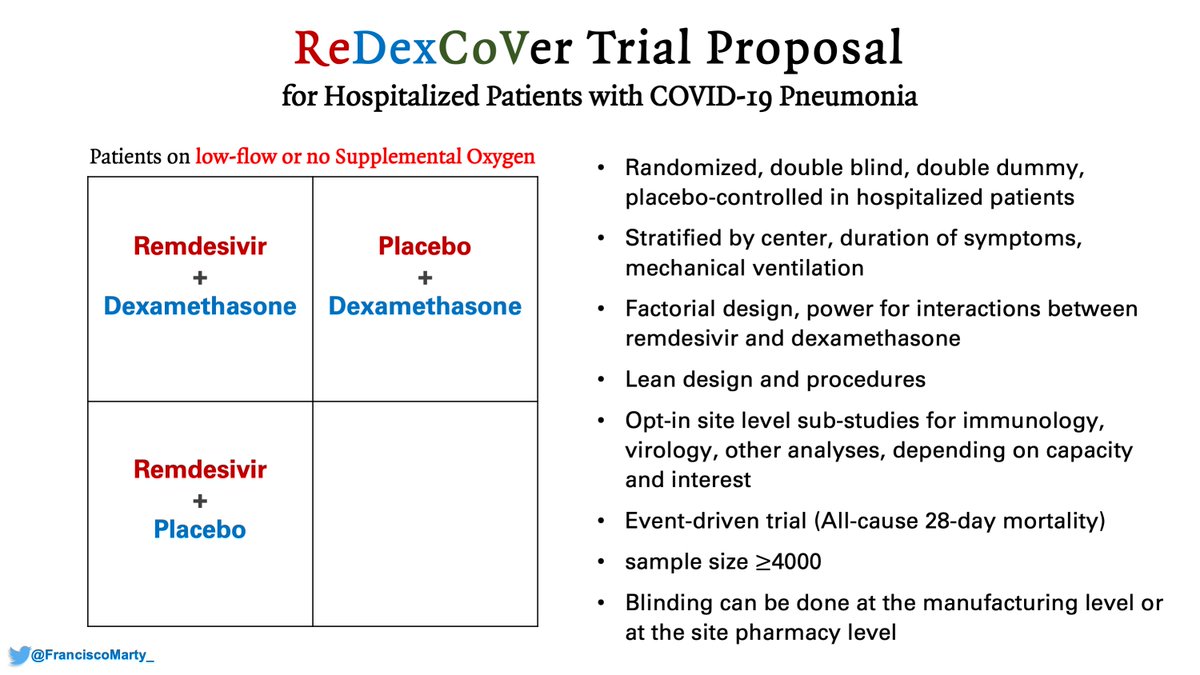
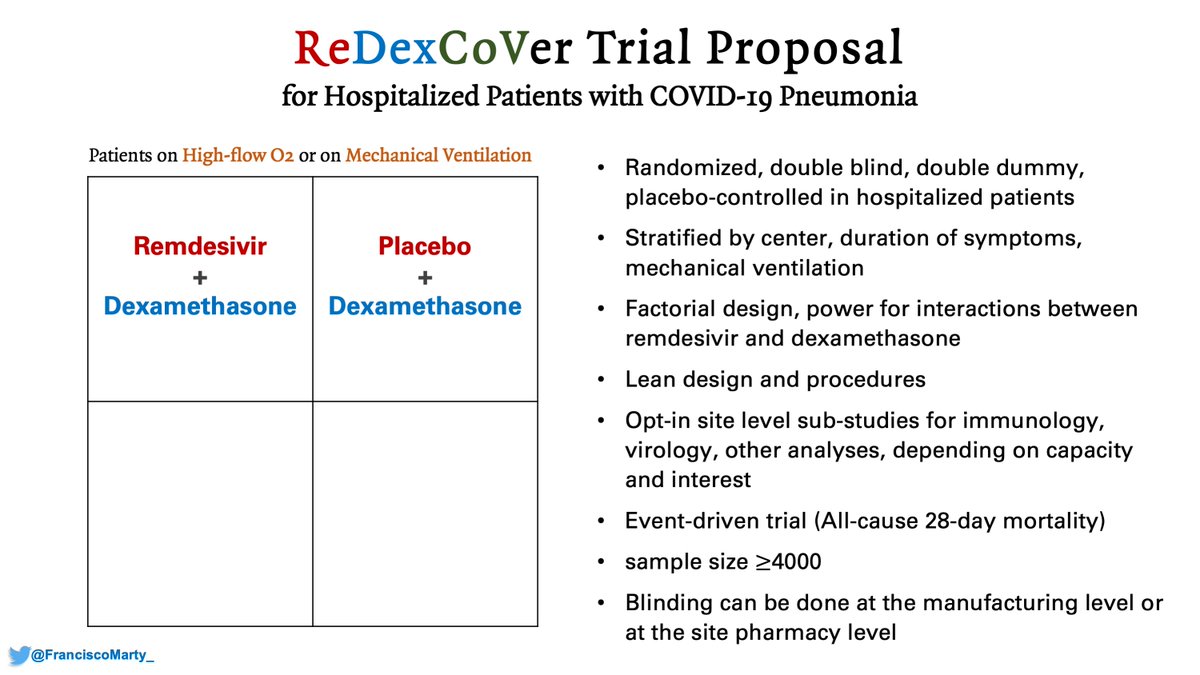
And then my pitch again for a confirmatory trial of #remdesivir and #dexamethasone, factorial design, run by ACTT or Sir Richard Peto's team, the #ReDexCoVer trial.

 Read on Twitter
Read on Twitter