If you've ever driven into Las Vegas from California, you passed by this epic field of ten thousand mirrors, each the size of a garage door.
Sunlight is concentrated to the top of a tower to heat molten nitrate salts over 900 degrees Fahrenheit.
Here's a thread on hot salt 1/n
Sunlight is concentrated to the top of a tower to heat molten nitrate salts over 900 degrees Fahrenheit.
Here's a thread on hot salt 1/n
Solar energy is stored in tanks of molten salt to power ~100,000 homes during the day and after the sun sets.
I think of it like a gigantic thermos. In the morning you fill up your thermos with hot coffee, and later in the afternoon when you need a boost in energy... 2/n
I think of it like a gigantic thermos. In the morning you fill up your thermos with hot coffee, and later in the afternoon when you need a boost in energy... 2/n
the hot coffee is there waiting patiently for you. Similarly the hot salt is stored in insulated steel tanks to stay hot at night time.
So when there is a demand for energy, heat is extracted from the molten salt to power turbines and generate clean electricity.
3/n
So when there is a demand for energy, heat is extracted from the molten salt to power turbines and generate clean electricity.
3/n
But how fast can you extract heat from molten nitrate salt? The thermal conductivity, aka the speed of heat, aka Yung Kelvin, will set the power limits of your heat exchanger and how much electricity can be produced.
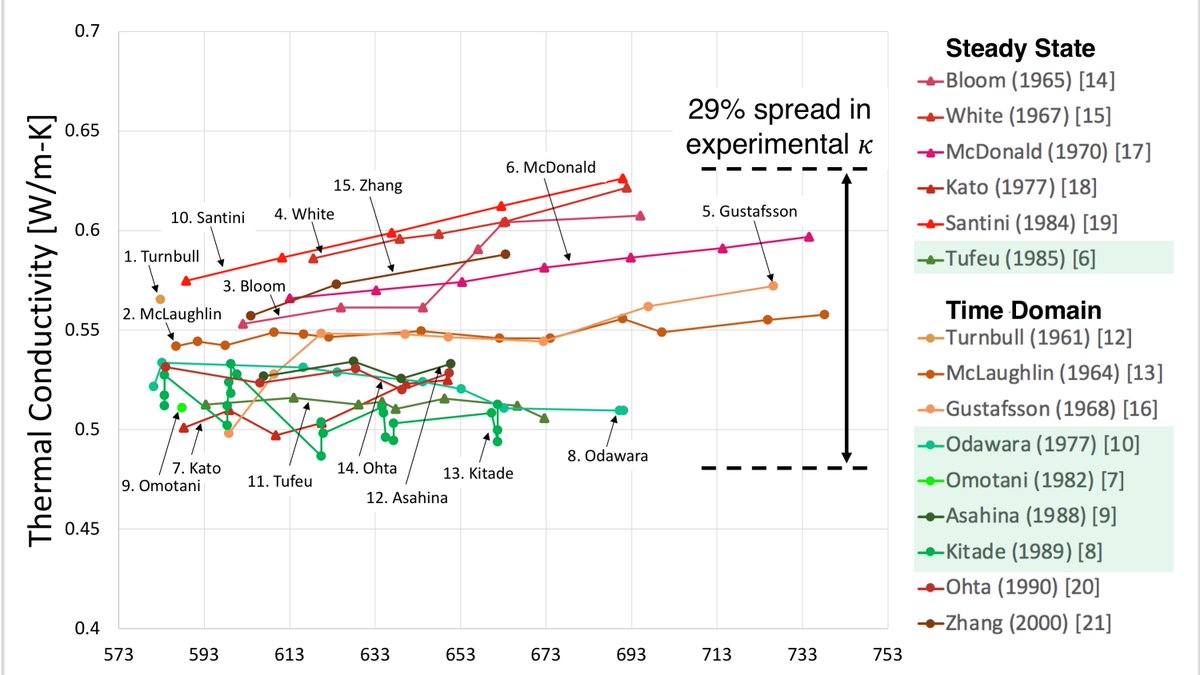
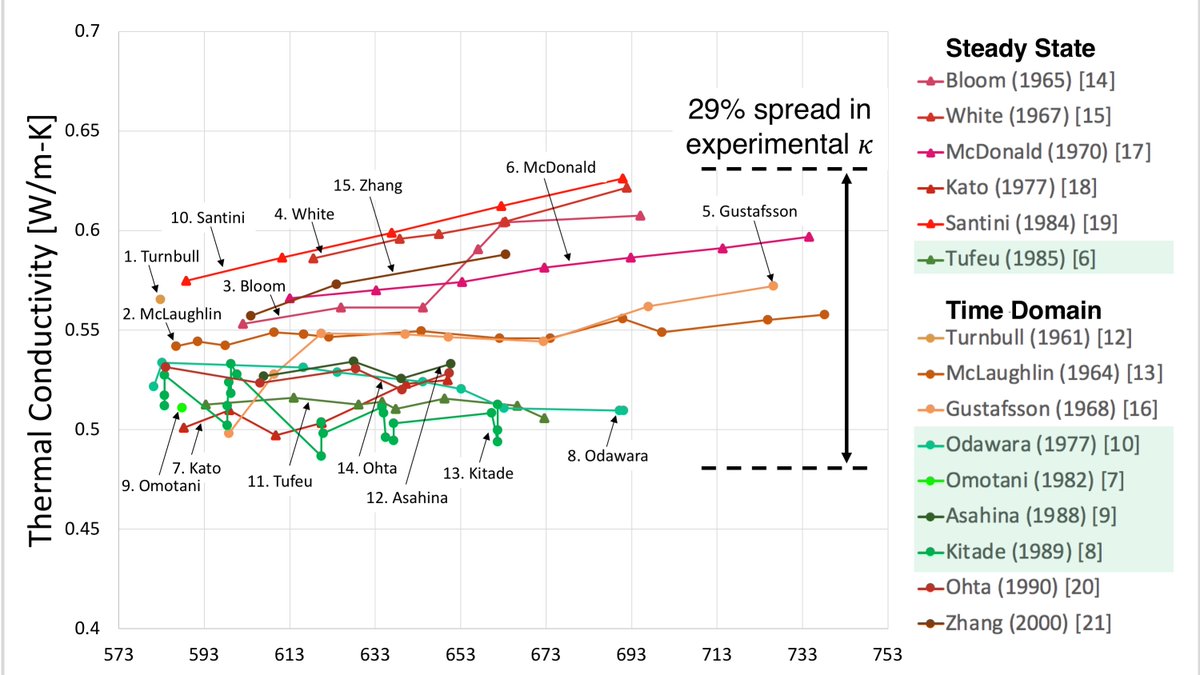
The thermal conductivity of molten sodium nitrate has been measured 15 times in the last 60 years, and this is what the data spread looks like this. From https://pubs.acs.org/doi/full/10.1021/acs.jced.0c00621
5/n
5/n
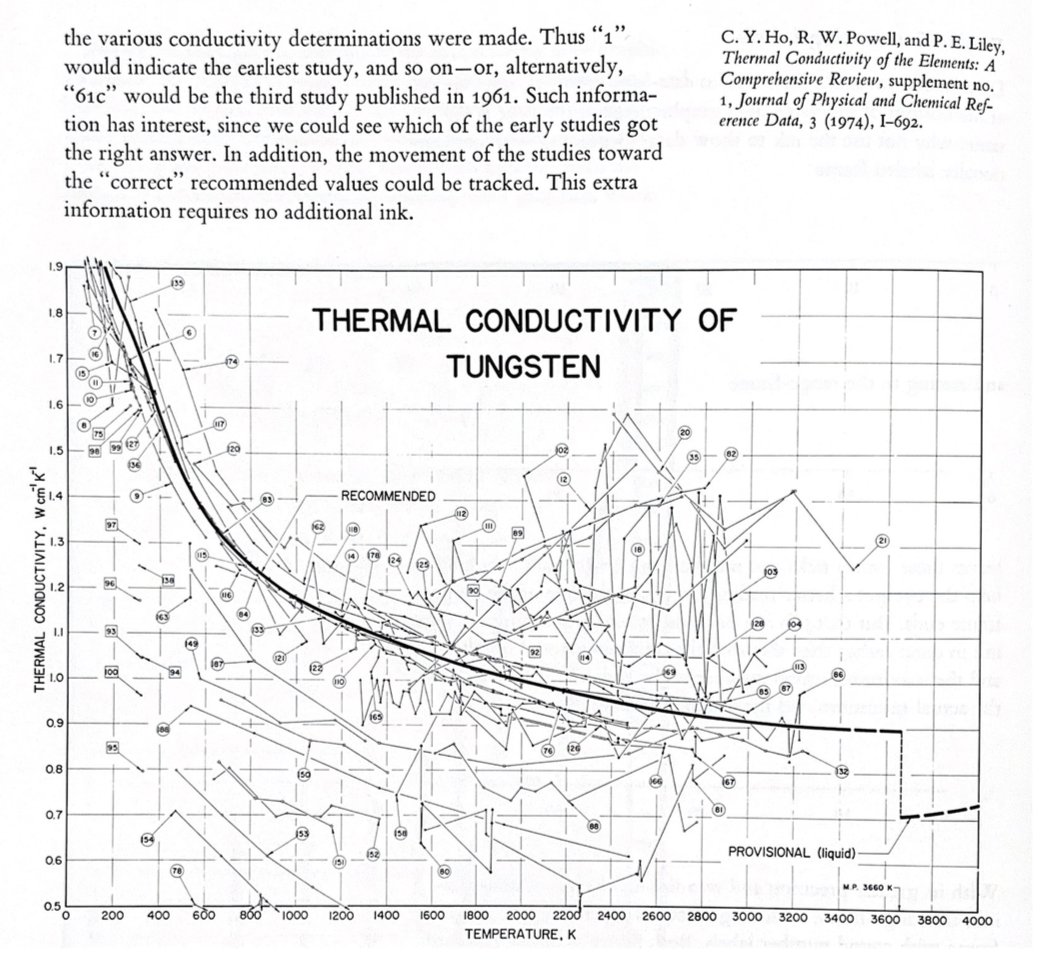
Side note the plot in tweet 5 was inspired by @EdwardTufte in The Visual Display of Quantitative Information. Where he noted the numbers in the figure for Tungsten (used in OG lightbulbs) could be numbered by publication date rather than alphabetically.
6/n
6/n
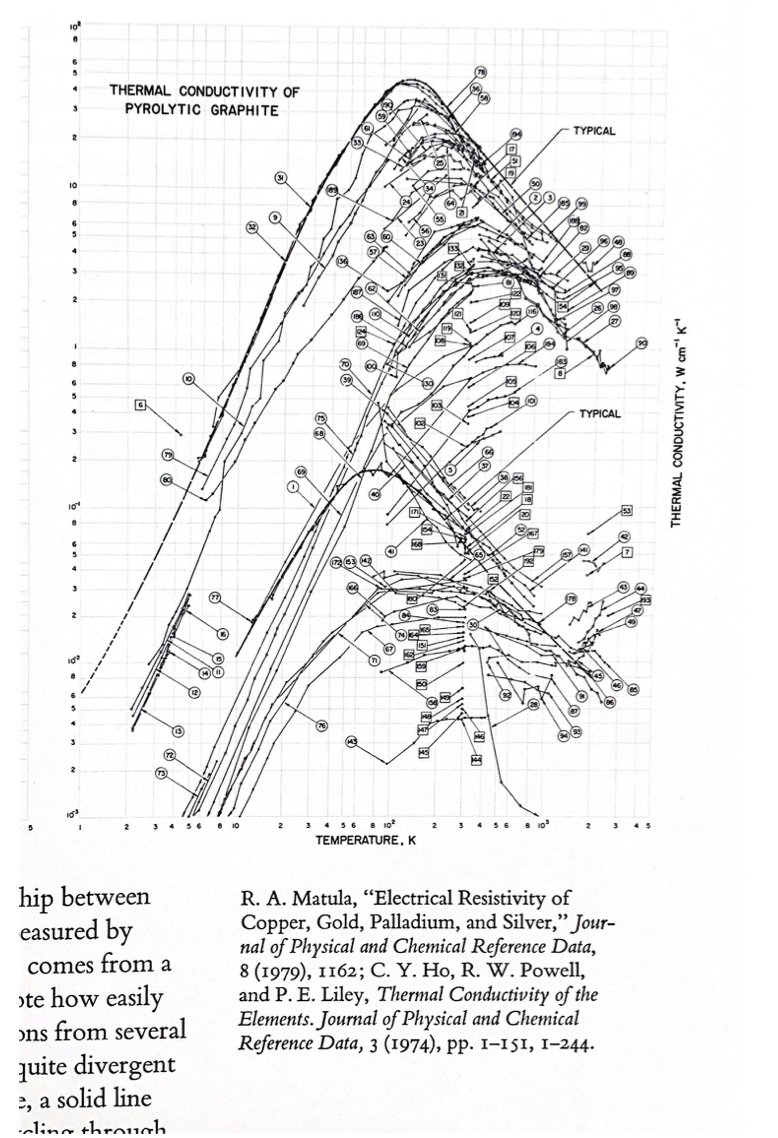
Also in Envisioning Information, @EdwardTufte makes one of the funniest comments about my field on page 39: "Note how easily these displays organize the material...from several hundred studies also enforcing comparisons among quite divergent results (this is science?)"
7/n
7/n
There are 2 traditional approaches to measure thermal conductivity. In both, you apply a known heat flux Q and then you either
1. measure the steady state temperature difference within the sample or
2. measure the temperature as a function of time.
1. measure the steady state temperature difference within the sample or
2. measure the temperature as a function of time.
You can read more about previous measurement techniques on the Wikipedia page, which I edited when I was procrastinating on writing my manuscript.
https://en.wikipedia.org/wiki/Thermal_conductivity_measurement
https://en.wikipedia.org/wiki/Thermal_conductivity_measurement
Amazing quote from @yicuistanford that I roughly remember from undergrad: "Wikipedia is a great resource to learn something new. I use it all the time, and whenever it's wrong I just correct it."
One of a million reasons I love Wikipedia.
10/n
One of a million reasons I love Wikipedia.
10/n
The main problem with traditional steady-state and time-domain techniques is that at really high temperatures, convection is magnified and you can't really measure the thermal conductivity. Which is what caused the large spread in previous data.
Essentially everyone built their own ovens to measure these molten salts, and it turned out - as any good baker knows - that the amount of convection changes from oven to oven.
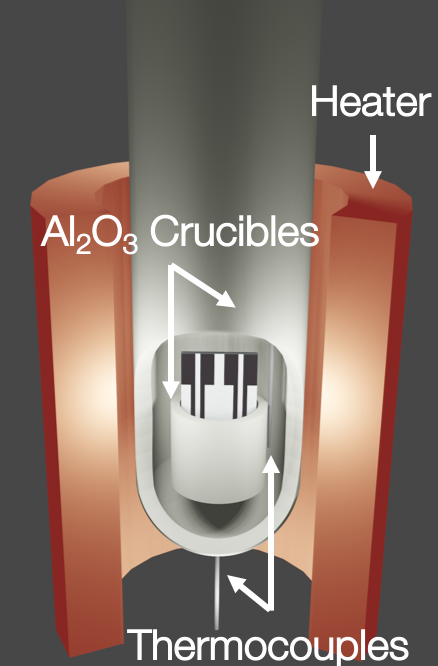
After Reviewer 2 asked what my oven looked like on the inside, I decided to learn blender after I saw https://twitter.com/DrewCoffman/status/1274743473732632576
I was like if Blender can animate Hollywood quality CGI scenes, I can render a few dumb rectangles and cylinders.
I was like if Blender can animate Hollywood quality CGI scenes, I can render a few dumb rectangles and cylinders.
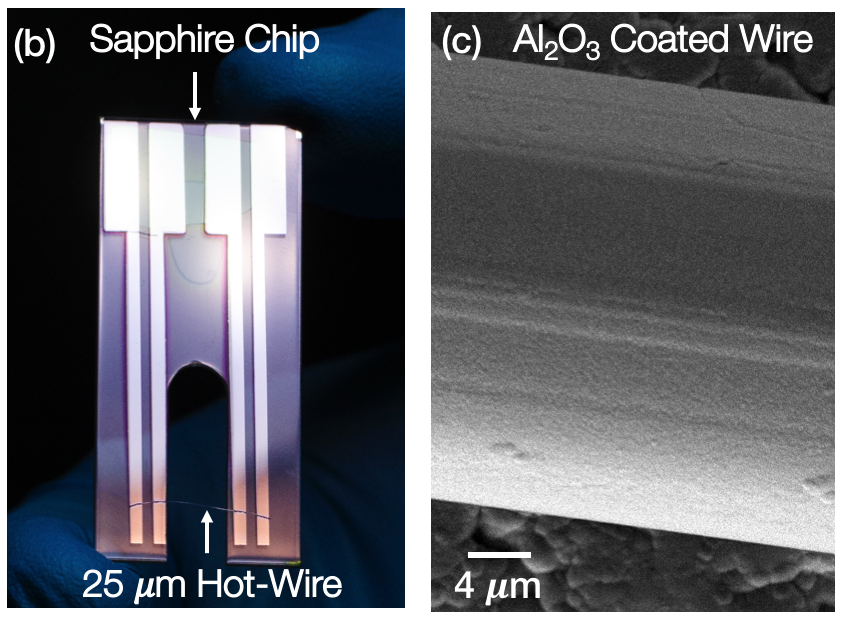
Also I made some jewelry quality sapphire and platinum sensors that can withstand corrosion from molten salt.
Also I've been beating around the bush.
The major innovation is using the ~Frequency-Domain~ to remove the error from convection.
AC current heats up the platinum wire to probe < 1 microliter of salt. The frequency varies from 1 beat per second to 1000 beats per second.
The major innovation is using the ~Frequency-Domain~ to remove the error from convection.
AC current heats up the platinum wire to probe < 1 microliter of salt. The frequency varies from 1 beat per second to 1000 beats per second.
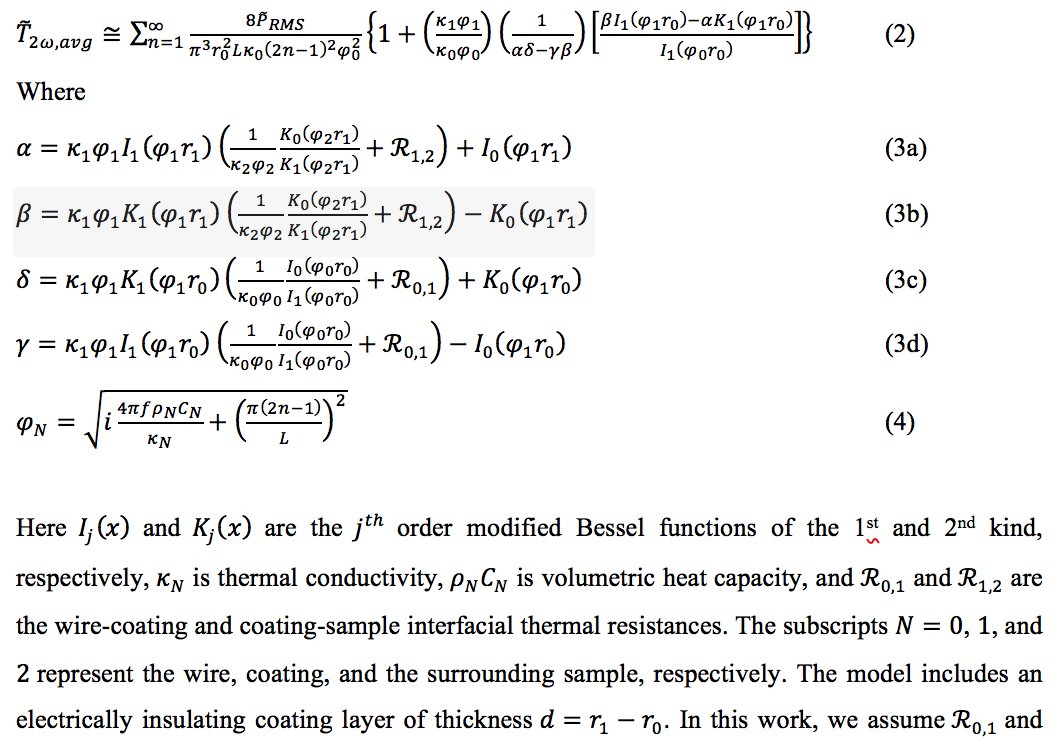
This is what the raw data looks like, which we fit to our thermal model shown in this ridiculously long, but good looking, equation.
From the measured data and model, we can extract the molten salt's thermal conductivity!
From the measured data and model, we can extract the molten salt's thermal conductivity!
If you compare our frequency-domain measurements to the previous reference correlation of the "best" steady-state and time-domain measurements, you can see a pretty big difference in values.
These measurements will be used in future designs of heat exchangers using molten nitrate salts.
Plus imma use my measurements to model molten chlorides and molten fluorides, which are gonna be used in even higher temperature, more efficient solar and nuclear power plants
19/n
Plus imma use my measurements to model molten chlorides and molten fluorides, which are gonna be used in even higher temperature, more efficient solar and nuclear power plants
19/n
Maybe if I spent less time on twitter, it would've taken me less than 4 years to build my oven and measure those dumb dots.
But it still feels good to get that first, first author publication off my back so I can move on with my life.
20/20
But it still feels good to get that first, first author publication off my back so I can move on with my life.
20/20

 Read on Twitter
Read on Twitter