Happy @US_FDA #VRBPAC briefing material release day for those crazy people like me who celebrate: https://www.fda.gov/advisory-committees/advisory-committee-calendar/vaccines-and-related-biological-products-advisory-committee-december-10-2020-meeting-announcement#event-materials
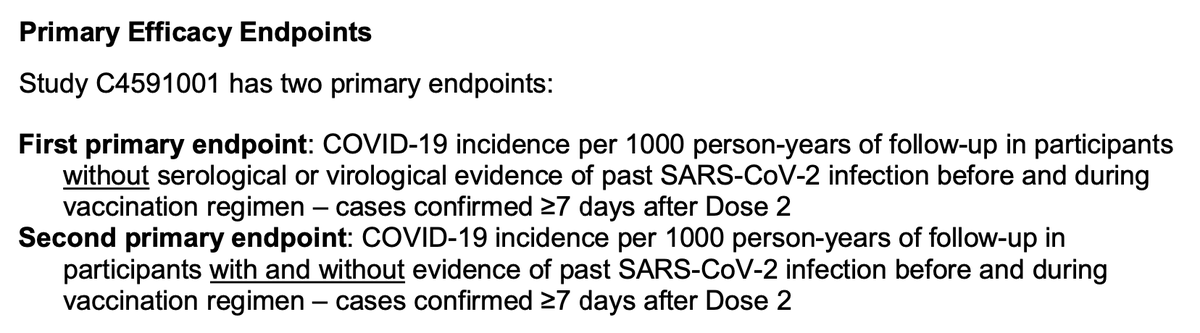
Some quick finds from the @US_FDA briefing materials: key to remember the primary endpoints for pivotal trial were examining incidence of #COVID19 disease - not asymptomatic PCR positivity or immunogenicity (antibody or T-cell), which are also of interest in different ways.
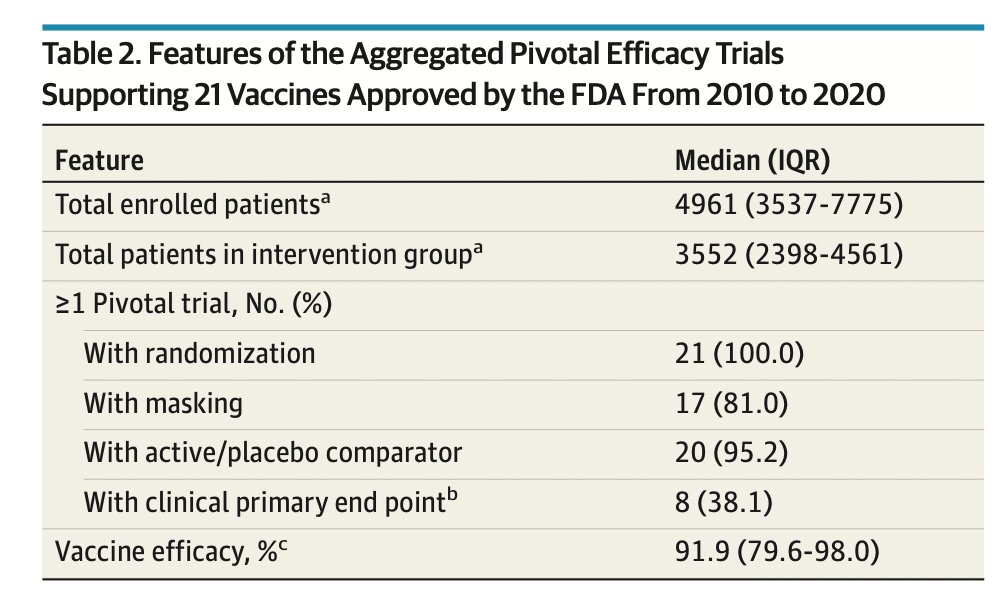
Also worth remembering the achievement it was to set up and enroll a trial of this size (>40,000!). In our study of vaccines approved in the last 10 years, we found median enrolled patients was around 5000: https://jamanetwork.com/journals/jamainternalmedicine/fullarticle/2772943
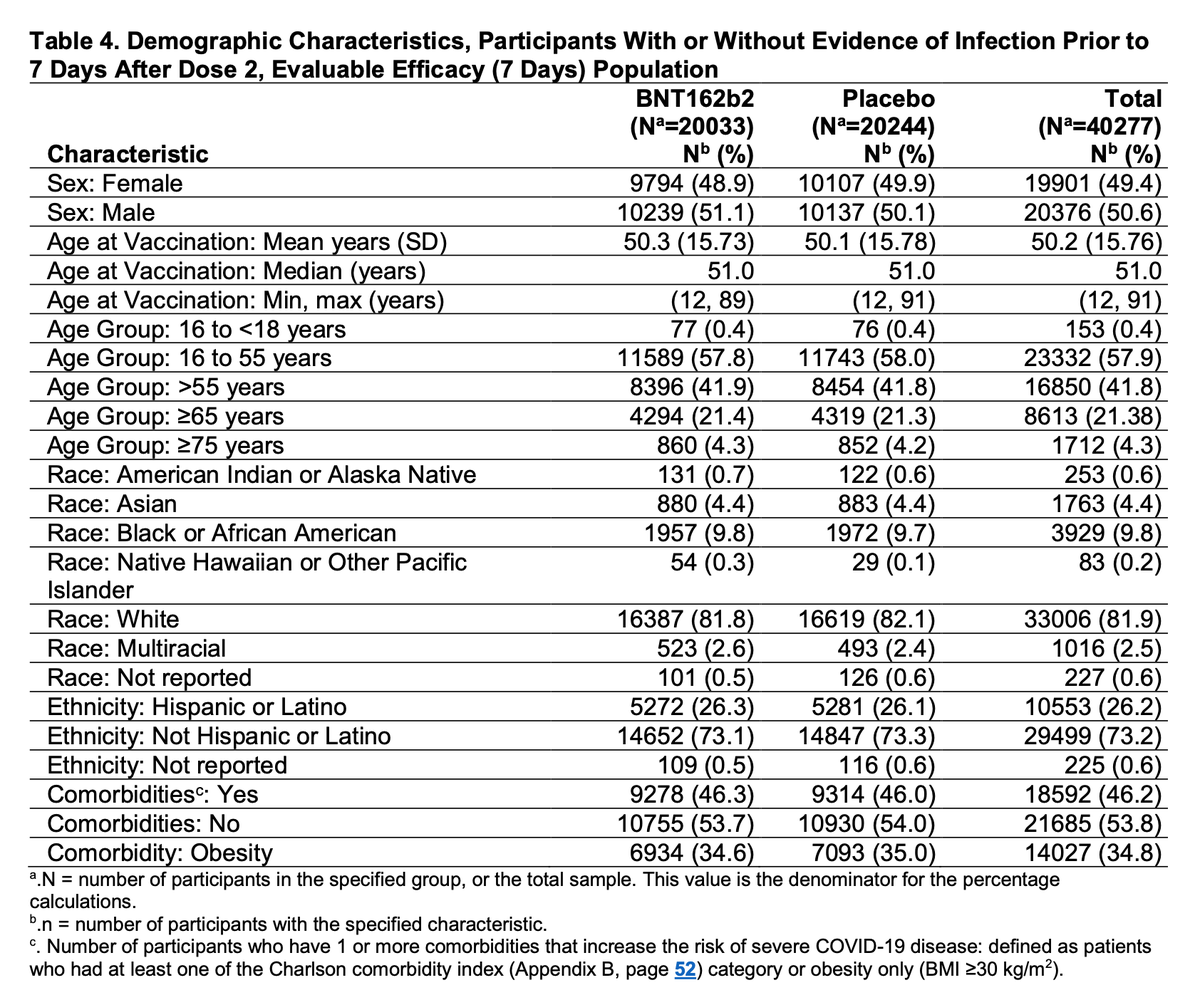
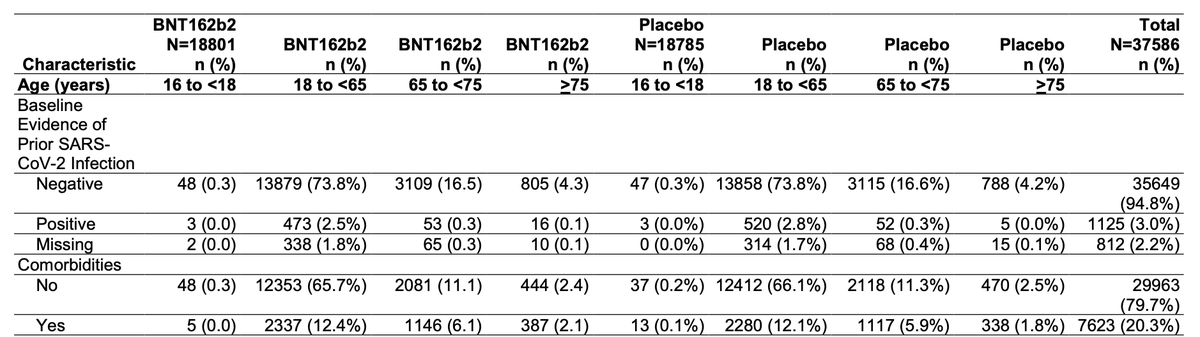
Pivotal trial demographics highlight efforts to enroll older adults, with >40% of evaluated population >55y. Still notable underrepresentation of Black participants (9.8% vs 13.4% US pop) though not Hispanic participants (26.2% vs 18.5% US pop).
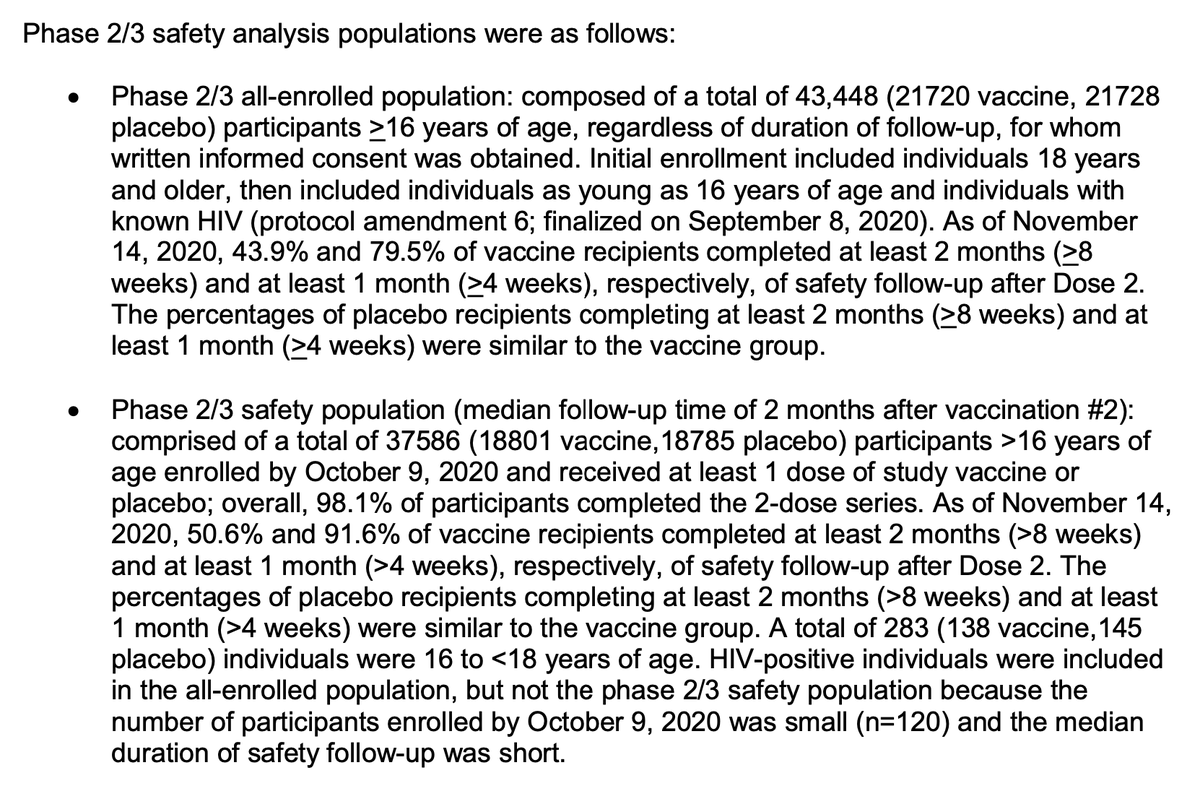
Inclusion of adults with comorbidities looks less impressive in the table of the safety population, which no longer counts obesity alone as a comorbidity, dropping the apparent rate of inclusion from 46.2% to 20.3%.
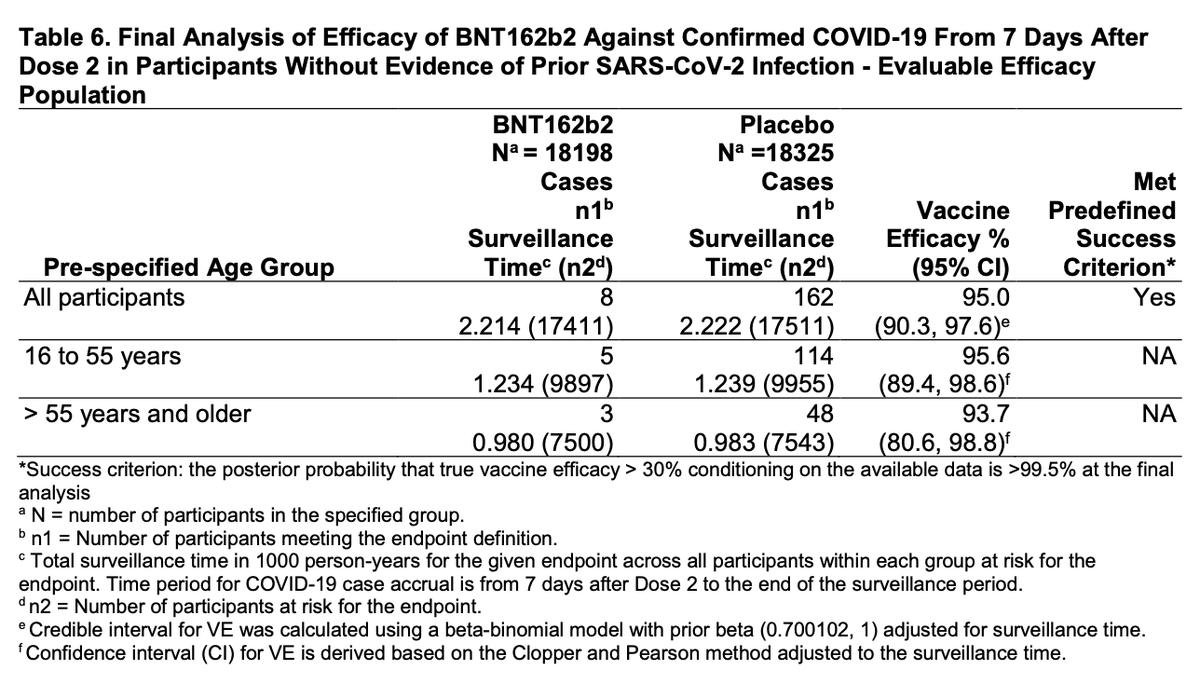
Analysis of efficacy at first glance looks strong in both middle-aged and older adults, as well as adults with certain comorbidities (though wide CIs in the subgroups).
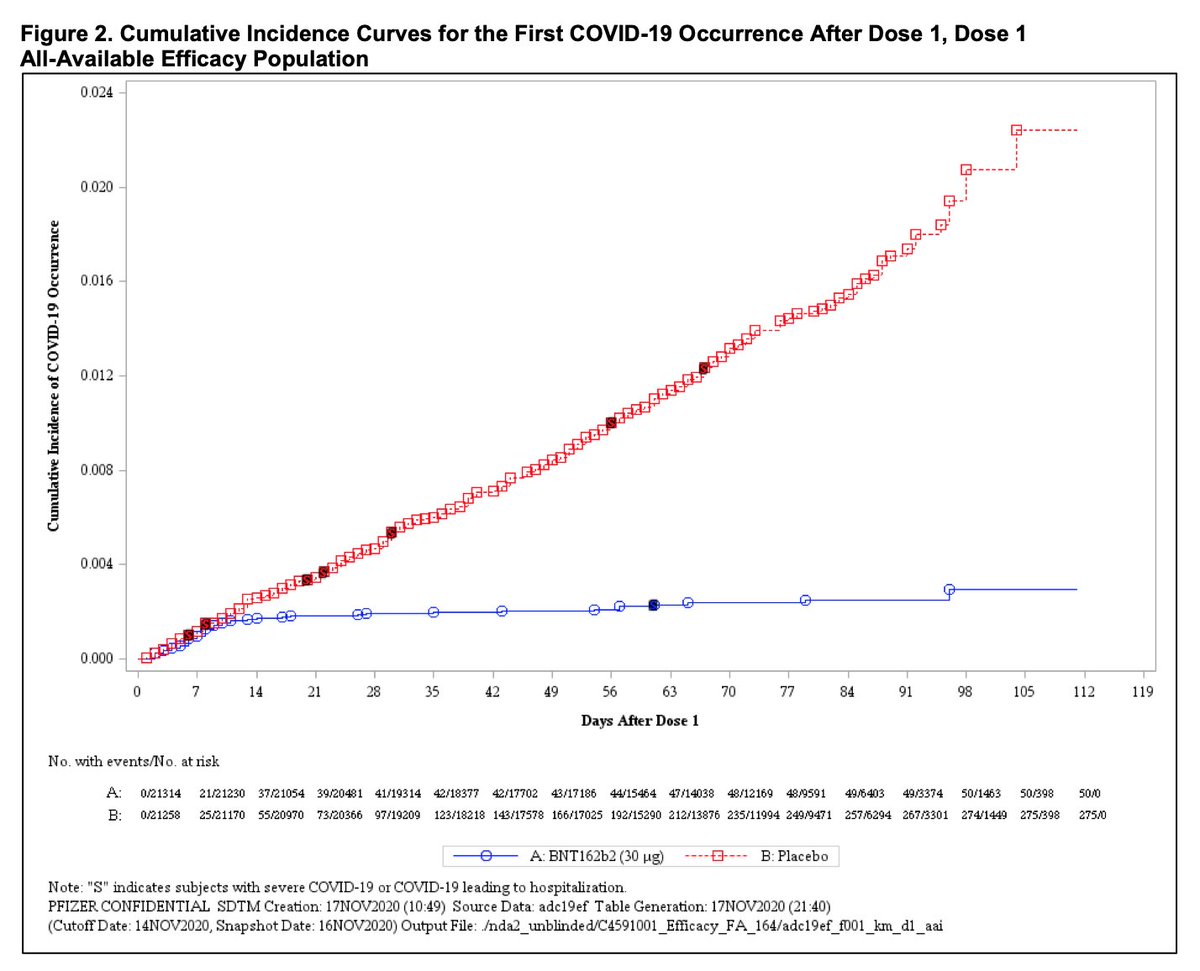
This figure potentially very promising as we consider the value of a single dose of a vaccine - incidence of #COVID19 begins to flatten after about 10-14 days after vaccine administration, though figure only tracks 4 month follow-up.
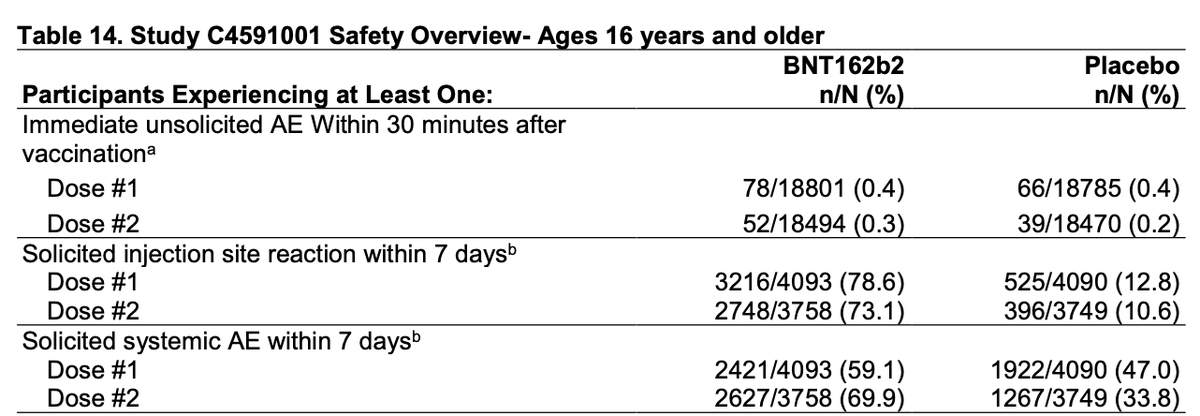
Something to note for vaccine rollout - the rate of local and systemic reactions after vaccination (though check out high rates of systemic reactions attributed to placebo as well).
Every year, so many are dissuaded from getting the flu vaccine because they remember when they "got the flu" (fever and chills) from it. Strong communication as to expected potential reactions from a #COVID19 vaccine will be critical for uptake during rollout.
2 deaths in the vaccine group and 4 in the placebo group - rates expected for the general population.
@DerekLowe has a nice blog post precisely about the errors of attribution we can expect to see on this front in the future: https://blogs.sciencemag.org/pipeline/archives/2020/12/04/get-ready-for-false-side-effects (via @HelenBranswell)
@DerekLowe has a nice blog post precisely about the errors of attribution we can expect to see on this front in the future: https://blogs.sciencemag.org/pipeline/archives/2020/12/04/get-ready-for-false-side-effects (via @HelenBranswell)
There is very limited data on vaccination around pregnancy (and no outcomes yet).
Recent pieces highlight that by excluding pregnant women (and others) from trials, we simply extend the period in which medical decision-making occurs in a data-free space: https://marlin-prod.literatumonline.com/pb-assets/Health%20Advance/journals/ajogmf/AJOG_COVID19_vaccine_in_pregnancy.pdf
Recent pieces highlight that by excluding pregnant women (and others) from trials, we simply extend the period in which medical decision-making occurs in a data-free space: https://marlin-prod.literatumonline.com/pb-assets/Health%20Advance/journals/ajogmf/AJOG_COVID19_vaccine_in_pregnancy.pdf
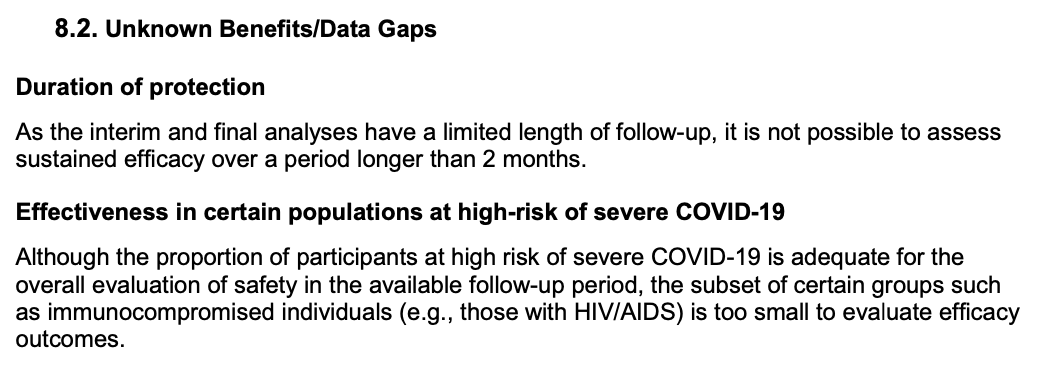
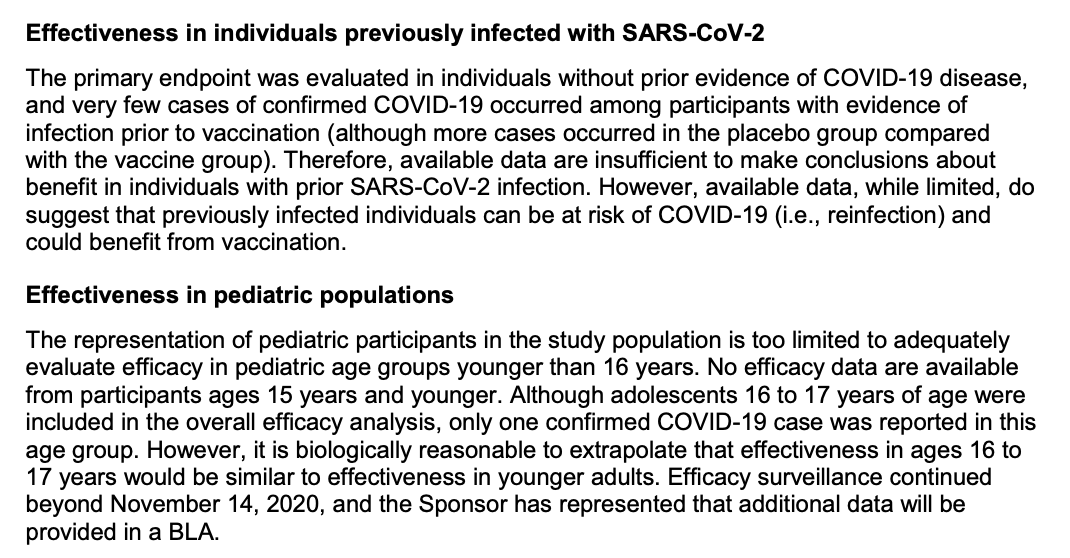
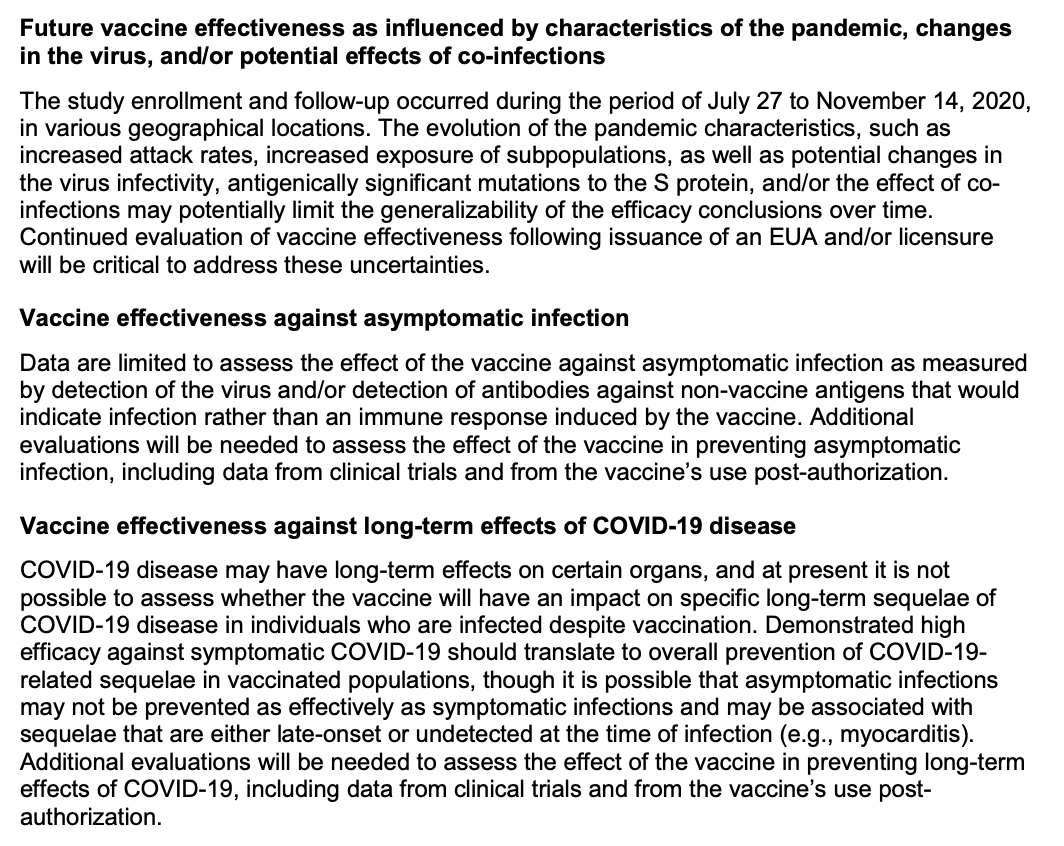
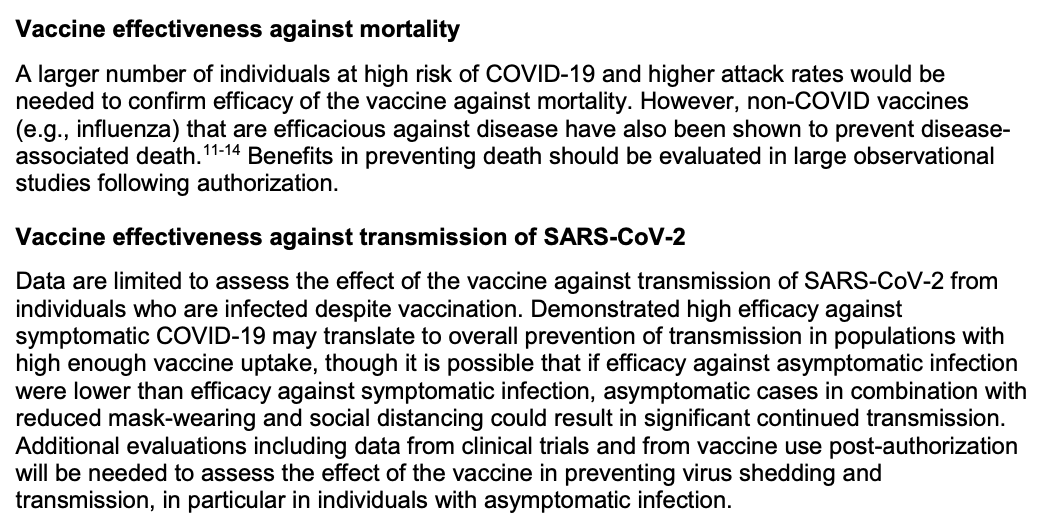
The trial leaves many unknowns regarding efficacy, including vaccine duration, effectiveness in certain populations, and effectiveness on endpoints not measured (including those critical to disease dynamics, like asymptomatic infection and transmission)...
... as well as the more popularly-discussed unknowns regarding safety, including safety in certain populations, or rare or longer-term adverse effects.
Signing off for now, but all materials publicly available on the @US_FDA website here: https://www.fda.gov/advisory-committees/advisory-committee-calendar/vaccines-and-related-biological-products-advisory-committee-december-10-2020-meeting-announcement#event-materials

 Read on Twitter
Read on Twitter