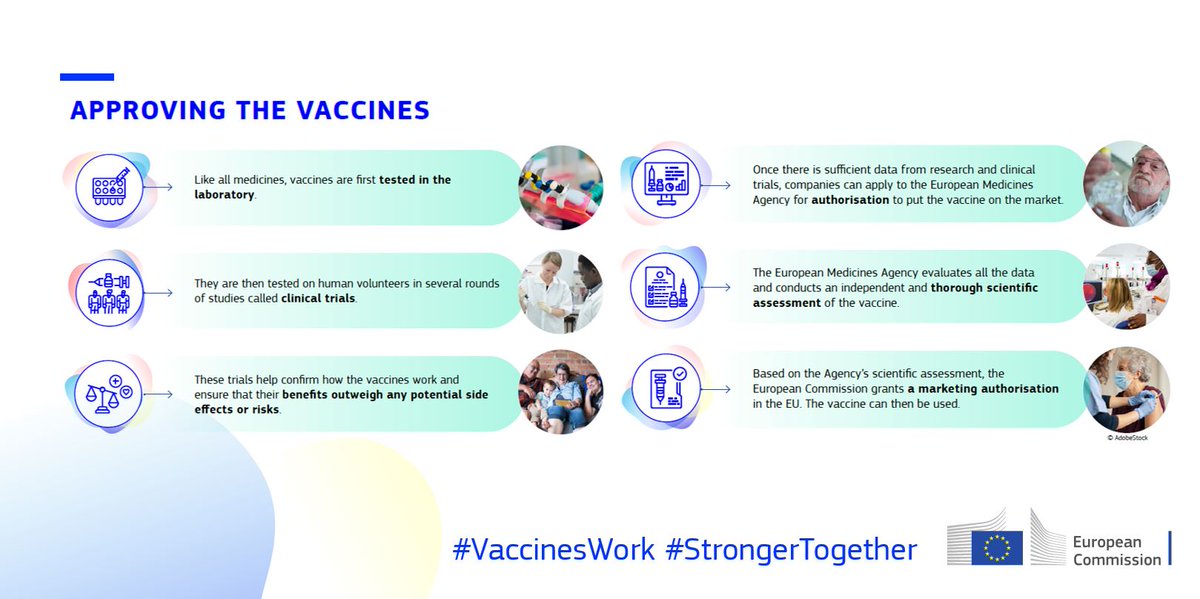
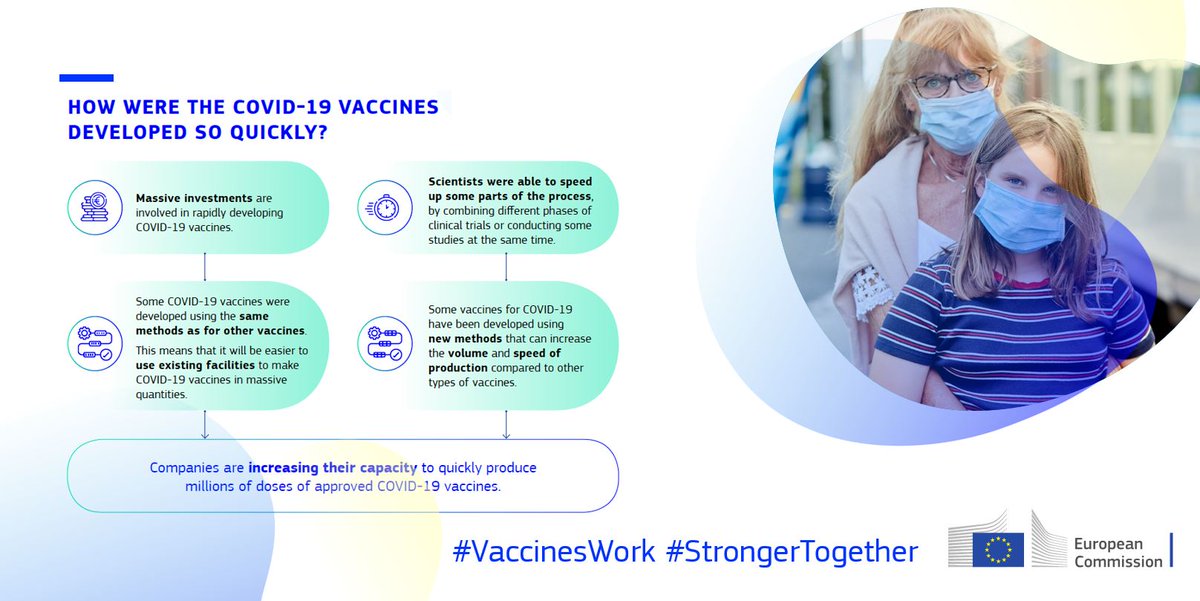
The EU has a rigorous authorisation process in place to ensure that any vaccine is safe and effective.
How are coronavirus vaccines developed, authorised
and put on the market?
Our thread #StrongerTogether #VaccinesWork
How are coronavirus vaccines developed, authorised
and put on the market?
Our thread #StrongerTogether #VaccinesWork
The EU's vaccine authorisation process includes:
 Laboratory tests
Laboratory tests
 Clinical trials
Clinical trials
 Scientific evaluation by @EMA_News
Scientific evaluation by @EMA_News
 Authorisation after consulting EU countries
Authorisation after consulting EU countries
#VaccinesWork #StrongerTogether
 Laboratory tests
Laboratory tests Clinical trials
Clinical trials Scientific evaluation by @EMA_News
Scientific evaluation by @EMA_News Authorisation after consulting EU countries
Authorisation after consulting EU countries#VaccinesWork #StrongerTogether
Coronavirus vaccines are being developed faster than usual, thanks to:
 Massive investments
Massive investments
 Use of existing facilities
Use of existing facilities
 Parallel work on different phases
Parallel work on different phases
 New working methods
New working methods
But they will meet the same high standards as other vaccines. #VaccinesWork #StrongerTogether
 Massive investments
Massive investments Use of existing facilities
Use of existing facilities Parallel work on different phases
Parallel work on different phases New working methods
New working methodsBut they will meet the same high standards as other vaccines. #VaccinesWork #StrongerTogether
We will only grant a marketing authorisation once the @EMA_News's assessment shows that the vaccine is both safe and effective.
Companies will then produce the approved vaccines on a large scale.
#VaccinesWork #StrongerTogether
Companies will then produce the approved vaccines on a large scale.
#VaccinesWork #StrongerTogether
The safety and effectiveness of vaccines are rigorously monitored, through the EU’s established medicines monitoring system.
The @EMA_News will set up additional large-scale safety monitoring for the coronavirus vaccine.
#VaccinesWork #StrongerTogether
The @EMA_News will set up additional large-scale safety monitoring for the coronavirus vaccine.
#VaccinesWork #StrongerTogether
Want to know more about how vaccines are authorised in the EU?
Hear directly from @EMA_News experts working on the approval of the coronavirus vaccines. They will discuss all the steps in this process.
 11 December: https://europa.eu/!Pc49kB
11 December: https://europa.eu/!Pc49kB
#VaccinesWork #StrongerTogether
Hear directly from @EMA_News experts working on the approval of the coronavirus vaccines. They will discuss all the steps in this process.
 11 December: https://europa.eu/!Pc49kB
11 December: https://europa.eu/!Pc49kB #VaccinesWork #StrongerTogether

 Read on Twitter
Read on Twitter