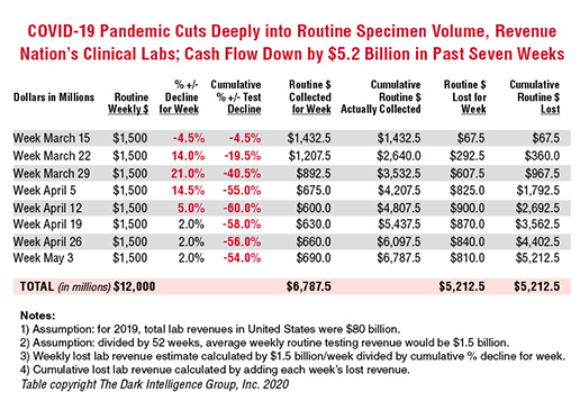
𝐃𝐞𝐯𝐢𝐥 𝐢𝐧 𝐭𝐡𝐞 𝐃𝐞𝐭𝐚𝐢𝐥𝐬
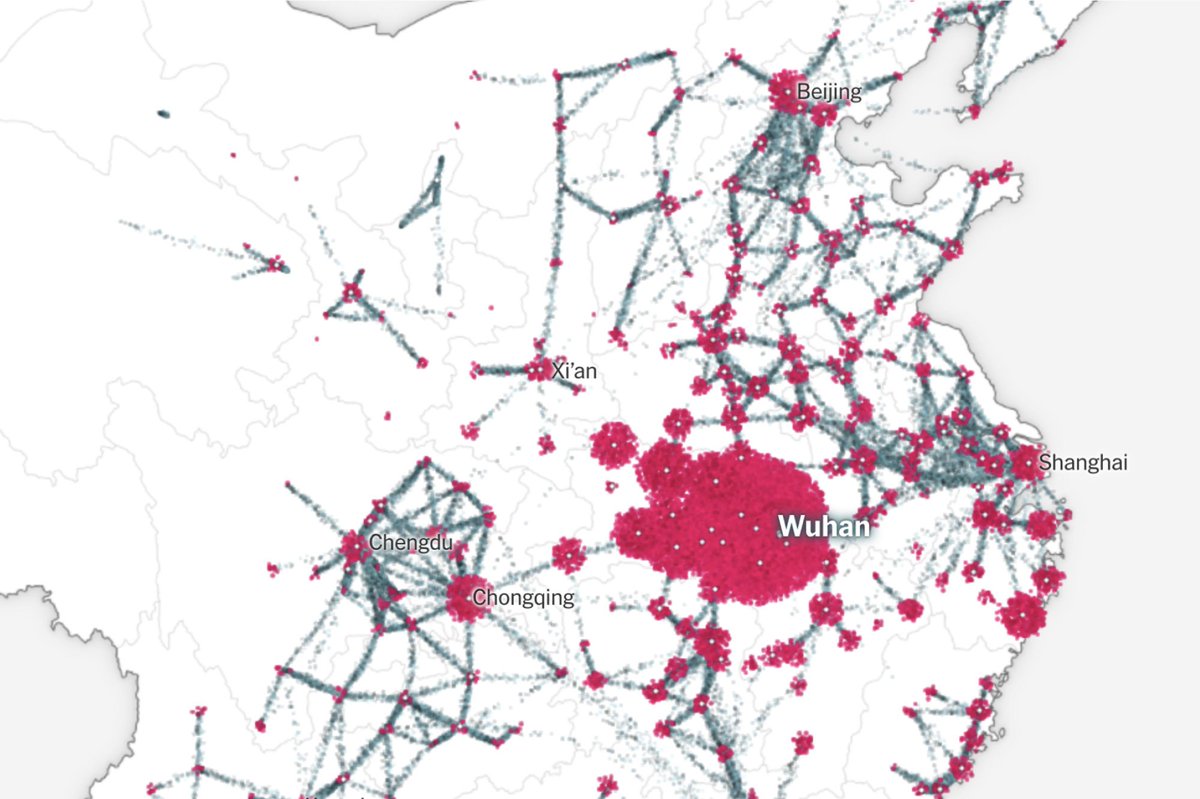
Starting late 2019, a coronavirus now dubbed SARS-CoV-2 emerged from China, becoming a pandemic that has forever altered the world's economic, cultural, and political landscape.
This is the story of the testing supply chain in the US.
1/
Starting late 2019, a coronavirus now dubbed SARS-CoV-2 emerged from China, becoming a pandemic that has forever altered the world's economic, cultural, and political landscape.
This is the story of the testing supply chain in the US.
1/
On Feb 4 2020, the US' Food and Drug Administration approved the first in vitro diagnostic test for the detection of COVID-19 (the sickness arising from infection with SARS-CoV-2).
This troubled test, submitted by the US' CDC, has nonetheless been the testing template.
2/
This troubled test, submitted by the US' CDC, has nonetheless been the testing template.
2/
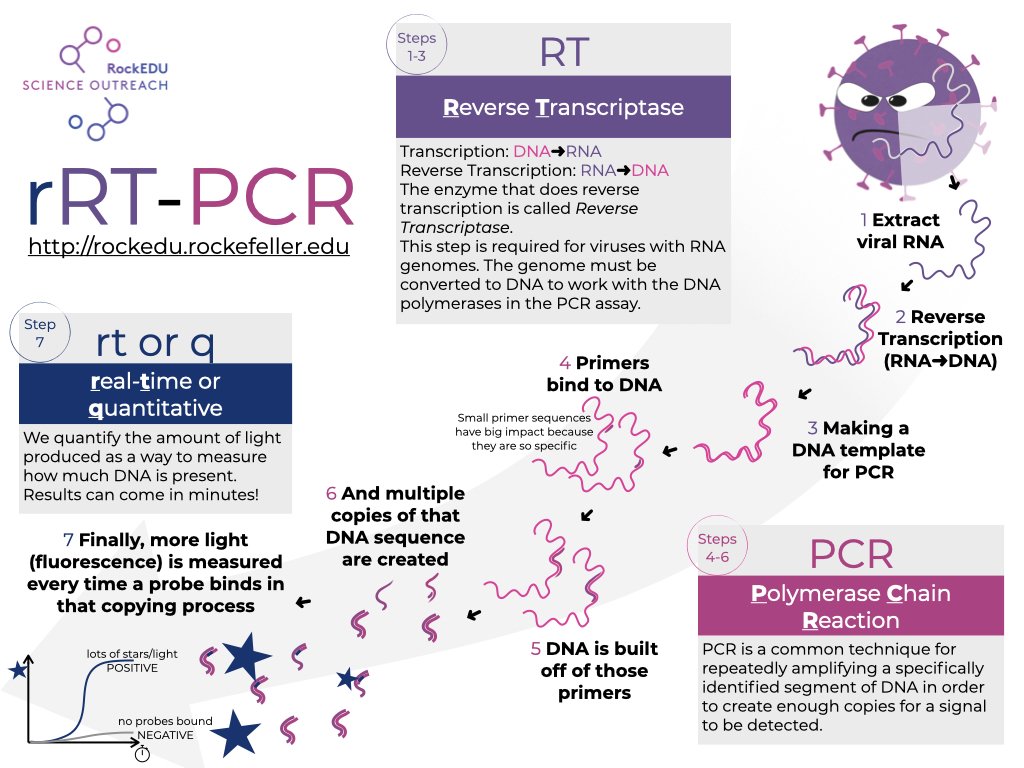
This test, like most offerings on the market, uses Reverse Transcription Polymerase Chain Reaction tests (RT-PCR).
It is a complex, but broadly accurate, testing methodology that utilizes a multi-step process to pinpoint specific traces of a virus in a specimen.
3/
It is a complex, but broadly accurate, testing methodology that utilizes a multi-step process to pinpoint specific traces of a virus in a specimen.
3/
Now, to Layman's Biology class for a moment:
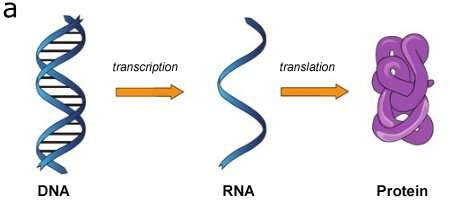
A genome is the complete set of RNA (ribonucleic acid) or DNA (deoxyribonucleic acid) information that comprises the "code" of an organism.
Normally, RNA creates proteins for cellular function according to instructions from DNA.
4/
A genome is the complete set of RNA (ribonucleic acid) or DNA (deoxyribonucleic acid) information that comprises the "code" of an organism.
Normally, RNA creates proteins for cellular function according to instructions from DNA.
4/
That process of "gene expression" is how DNA builds a living thing - the RNA tells the ribosomes what to do, which in turn use amino acids to build proteins.
The RNA is the bridge, the middleman.
Note, a type of RNA, messenger RNA (mRNA), will become key later.
5/
The RNA is the bridge, the middleman.
Note, a type of RNA, messenger RNA (mRNA), will become key later.
5/
This brings us to SARS-CoV-2. It's an "RNA virus", which means it doesn't operate as messenger for more complex DNA instructions.
All of its "data" is encoded as RNA - simply put, it's purpose-built solely to hijack our cells and replicate itself to cause sickness.
6/
All of its "data" is encoded as RNA - simply put, it's purpose-built solely to hijack our cells and replicate itself to cause sickness.
6/
Circling back to RT-PCR testing now...
PCR testing can only detect and analyze DNA, not RNA. Thus, SARS-CoV-2 is "unreadable" in its natural form.
The genetic material must undergo "reverse transcription", where the RNA is converted into complementary DNA (cDNA).
7/
PCR testing can only detect and analyze DNA, not RNA. Thus, SARS-CoV-2 is "unreadable" in its natural form.
The genetic material must undergo "reverse transcription", where the RNA is converted into complementary DNA (cDNA).
7/
Reverse Transcription is achieved by using an enzyme called "reverse transcriptase".
The RNA sample is run through the RT process, which generates a new sample containing cDNA that can be read by the actual PCR machine.
It's tedious work with no margin for error.
8/
The RNA sample is run through the RT process, which generates a new sample containing cDNA that can be read by the actual PCR machine.
It's tedious work with no margin for error.
8/
The vast majority of tests in use require that the material generated in the RT phase of sample prep be transferred to a new "plate" for the PCR test itself.
If this transfer ("pipetting") is done manually, there's a higher chance of contamination between all the samples.
9/
If this transfer ("pipetting") is done manually, there's a higher chance of contamination between all the samples.
9/
To reduce error, manufacturers like Eppendorf have developed fluid-handling robots that will pipette at precise tolerances far better than a human can achieve.
These are expensive machines, and have been backordered globally for months.
Thus, most labs are manual.
10/
These are expensive machines, and have been backordered globally for months.
Thus, most labs are manual.
10/
At the end of this production line are the PCR machines themselves.
Companies like Roche, Agilent, Thermo Fisher, Quiagen, and Bio-Rad normally don't see huge demand for such a relatively-niche product.
The explosion in clinical lab capacity has crushed the supply chains.
11/
Companies like Roche, Agilent, Thermo Fisher, Quiagen, and Bio-Rad normally don't see huge demand for such a relatively-niche product.
The explosion in clinical lab capacity has crushed the supply chains.
11/
Complicating matters are that nearly all of these machines, tests, and disposable components - trays, pipette tips, reagents - are manufactured overseas, from globally-sourced parts (many of them from China).
Everything is backordered, unless you pay up bigly.
12/
Everything is backordered, unless you pay up bigly.
12/
Then we come to the labs themselves. Reliable data is hard to come by, but from firsthand experience supplying >30 labs (and growing) around the country with all machines, tests, and components, I can say that the vast majority were not in business before March 2020.
13/
13/
At the onset of the pandemic when it became clear testing was a growth industry, many investors jumped in.
They rented office space, hired a lab manager, and began selecting and sourcing tests and equipment.
Important to note, most of them had no idea what they were doing.
14/
They rented office space, hired a lab manager, and began selecting and sourcing tests and equipment.
Important to note, most of them had no idea what they were doing.
14/
The established clinical lab networks like LabCorp, Sanofi, Abbott, and Quest all began pushing out their own tests that had been rushed into production and received Emergency Use Authorizations from FDA.
However, all other sampling stopped, crashing revenues.
15/
However, all other sampling stopped, crashing revenues.
15/
By mid-year, the US found itself trying to scale testing for a novel virus that originated in a nation famous for lying and opacity, without the number of qualified personnel or available equipment to meet demand, and a testing methodology that is notoriously finnicky.
16/
16/
This was a toxic brew. The labs, the test and equipment manufacturers, and the political apparatus all had vested interested in scaling testing as fast as possible pre-autumn.
Investors and companies needed ROI, and the politicians wanted control.
17/
Investors and companies needed ROI, and the politicians wanted control.
17/
However, the vertical growth in capacity introduced a new dynamic:
Standardization of test results across even a small population sample became impossible.
This is due to the variety of tests, methodologies, machines, and quality of personnel - even within a single city.
18/
Standardization of test results across even a small population sample became impossible.
This is due to the variety of tests, methodologies, machines, and quality of personnel - even within a single city.
18/
Further confusing to the layman is the question of exactly what constitutes a positive diagnosis.
A PCR machine functions by heating the cDNA sample multiple times, causing the DNA to separate. An enzyme ("polymerase") is introduced, which replicates the DNA strand.
19/
A PCR machine functions by heating the cDNA sample multiple times, causing the DNA to separate. An enzyme ("polymerase") is introduced, which replicates the DNA strand.
19/
This process (called "amplification") is repeated anywhere from 20-40+ times, producing hundreds of millions of copies of the original tiny cDNA sample.
In this way, the machine and operator are able to create a reliable pool of viral DNA to test.
20/
In this way, the machine and operator are able to create a reliable pool of viral DNA to test.
20/
However, the number of cycles ("Ct", or cycle threshold) is directly correlated to the quantity of viral RNA present in the original sample.
The higher the Ct value - the more copies of DNA that had to be created before detecting the virus - the less viral RNA present.
21/
The higher the Ct value - the more copies of DNA that had to be created before detecting the virus - the less viral RNA present.
21/
Similarly, the lower the Ct value, the more "sick" the patient is with SARS-CoV-2, because fewer amplification cycles were required before detecting the virus.
If the testing reaches the cycle limit - usually 37 to 40 - without detecting the virus, it is negative.
22/
If the testing reaches the cycle limit - usually 37 to 40 - without detecting the virus, it is negative.
22/
The New York Times ably reported on the issue of Ct values back on August 29, raising hairs on the heads of people who suspected nat'l data is too opaque to be making draconian edicts and policies.
Notably, the FDA refuses to set a nat'l standard.
23/ https://www.nytimes.com/2020/08/29/health/coronavirus-testing.html
Notably, the FDA refuses to set a nat'l standard.
23/ https://www.nytimes.com/2020/08/29/health/coronavirus-testing.html
Yet while the issue of Ct values is extremely important to the overall policy debate and political narratives, there are more fundamental chokepoints in the US' testing ecosystem.
First is the issue of overabundance of test kits.
To date, the FDA has issued 196 EUA's.
24/
First is the issue of overabundance of test kits.
To date, the FDA has issued 196 EUA's.
24/
Some of these EUA's supersede earlier iterations of a manufacturer's test, such as upgrading from a monoplex to multiplex test.
However, the number of active tests on the market easily exceed 150, manufactured in China, USA, Europe, Turkey, Korea, and elsewhere.
25/
However, the number of active tests on the market easily exceed 150, manufactured in China, USA, Europe, Turkey, Korea, and elsewhere.
25/
A standard SARS-CoV-2 PCR test kit will come with enough reagent to perform 50-100 tests.
Nearly all require constant -20C temp control (a normal freezer is adequate). The enzymes and reagents destabilize after 5-14+ days outside of this temp range, depending on the kit.
26/
Nearly all require constant -20C temp control (a normal freezer is adequate). The enzymes and reagents destabilize after 5-14+ days outside of this temp range, depending on the kit.
26/
The kits are small, though, and many can be shipped in a single insulated shipper box with ice packs or dry ice blocks/pellets.
Thus, a seller might have up to $100,000 worth of test chemicals relying on the logistics vendors to responsibly handle and monitor one small box.
27/
Thus, a seller might have up to $100,000 worth of test chemicals relying on the logistics vendors to responsibly handle and monitor one small box.
27/
That's a huge risk of loss due to damage to the foam box from carelessness, load shift in transit, or rough handling in the sortation centers of UPS, FedEx, DHL, etc.
I've personally seen boxes arrive damaged to my own warehouse with tape over holes and warm kits inside.
28/
I've personally seen boxes arrive damaged to my own warehouse with tape over holes and warm kits inside.
28/
Why is this a chokepoint?
Because a lab cannot tell if the enzymes or reagent have destabilized until they run the tests, and no amplification occurs.
A new batch of samples must be prepared from scratch and re-run.
Transport is essentially an honor system by the seller.
29/
Because a lab cannot tell if the enzymes or reagent have destabilized until they run the tests, and no amplification occurs.
A new batch of samples must be prepared from scratch and re-run.
Transport is essentially an honor system by the seller.
29/
Best practice for cold-chain transport of things like PCR test kits (or vaccines) is to have constant, auditable temp monitoring during storage and transport.
But right now, with demand sky high, labs are hand to mouth on supply. Every test not run is $100+ in lost revenue.
30/
But right now, with demand sky high, labs are hand to mouth on supply. Every test not run is $100+ in lost revenue.
30/
Once a manufacturer/seller has a lab client validated on a specific test/equipment combination, it becomes difficult and costly for the lab to switch.
And given that many of the testing labs (and test providers) did not exist a year ago, things get overlooked.
31/
And given that many of the testing labs (and test providers) did not exist a year ago, things get overlooked.
31/
In any supply chain operation, there are tradeoffs between performance and cost efficiency.
Quite simply, the incentive is on the lab to assume with minimal auditing that the suppliers and transport vendors have been perfect.
Receive tests, use tests, provide results.
32/
Quite simply, the incentive is on the lab to assume with minimal auditing that the suppliers and transport vendors have been perfect.
Receive tests, use tests, provide results.
32/
Regulatory burdens on imports is another bottleneck.
For months, the FDA and Customs and Border Protection agencies struggled to process SARS-CoV-2 test imports in a timely manner.
Remember - these are cold chain, airfreighted, requiring constant attention.
33/
For months, the FDA and Customs and Border Protection agencies struggled to process SARS-CoV-2 test imports in a timely manner.
Remember - these are cold chain, airfreighted, requiring constant attention.
33/
But, here's the rub:
The customs clearance (and FDA) process is governed by Harmonized Tariff System codes (HTS).
These numbers specify exactly what the item is, applicable duties, and trigger alerts to other agencies that may have oversight.
They cannot be lied about.
34/
The customs clearance (and FDA) process is governed by Harmonized Tariff System codes (HTS).
These numbers specify exactly what the item is, applicable duties, and trigger alerts to other agencies that may have oversight.
They cannot be lied about.
34/
In the case of PCR test kit HTS code (3822.00), filing a customs entry will "flag" CBP to send the import record to FDA for review.
FDA entries have their own codes that must be entered alongside the HTS code. An FDA official then reviews the entry for legality.
35/
FDA entries have their own codes that must be entered alongside the HTS code. An FDA official then reviews the entry for legality.
35/
However, there is no correct way to submit an entry for PCR test kits imported under an EUA from FDA.
Test kits ordinarily go through a process of approvals, at which time a registration number is assigned and used to import. An EUA breaks this.
No number means "red flag".
36/
Test kits ordinarily go through a process of approvals, at which time a registration number is assigned and used to import. An EUA breaks this.
No number means "red flag".
36/
A red flag in the FDA entry review process means extra scrutiny and extra delays.
A good customs broker has to resolve this by submitting all paperwork (EUA letter, etc) at the time of entry and "spoofing" the entry with incorrect data to get it even submitted for review.
37/
A good customs broker has to resolve this by submitting all paperwork (EUA letter, etc) at the time of entry and "spoofing" the entry with incorrect data to get it even submitted for review.
37/
Not every customs broker is a good one.
Two of the Big Three parcel companies have completely screwed up customs and FDA clearance for my own customer to the point of me threatening legal action just to get them to re-ice my boxes to prevent spoilage.
38/
Two of the Big Three parcel companies have completely screwed up customs and FDA clearance for my own customer to the point of me threatening legal action just to get them to re-ice my boxes to prevent spoilage.
38/
In response, I had to completely rebuild the import process such that these carriers have zero involvement.
Yet, it is these same carriers that many of the test kit importers must rely on, because they do not understand international logistics at a granular level.
39/
Yet, it is these same carriers that many of the test kit importers must rely on, because they do not understand international logistics at a granular level.
39/
These delays cause all manner of downstream issues to test kit importers and labs, as many shipments of product reach the end of their "safe" timetable for cold chain without having been delivered or managed properly.
Do they dispose, or risk bad/ineffective tests?
40/
Do they dispose, or risk bad/ineffective tests?
40/
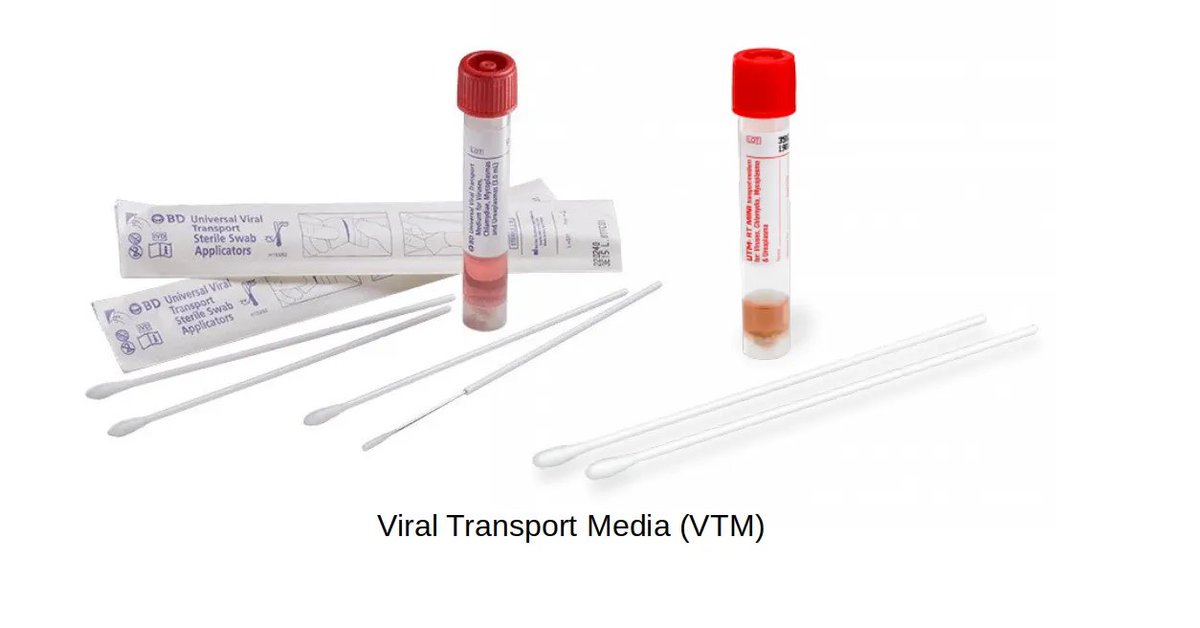
One final chokepoint to note is that of VTM's.
Viral Transport Media are used to safely store and transport specimens on swabs from the testing site to the lab running the test.
There are numerous manufacturers, most with one story to tell:
They can't keep up.
41/
Viral Transport Media are used to safely store and transport specimens on swabs from the testing site to the lab running the test.
There are numerous manufacturers, most with one story to tell:
They can't keep up.
41/
Demand for the swabs and tubes has exploded, with new capacity slower to come online.
Prices are increasing quickly. I've observed a 15% increase in two months from my customer's two primary suppliers.
With ongoing airfreight capacity issues, shipping is delayed as well.
42/
Prices are increasing quickly. I've observed a 15% increase in two months from my customer's two primary suppliers.
With ongoing airfreight capacity issues, shipping is delayed as well.
42/
Adding it all up, the US is making policy based on *non-standardized testing* in an industry suffering from:
- Over-sensitive testing methodology
- Major logistics constraints
- Opaque/shifting regulatory reqs
- Inexperienced vendors and stakeholders
- Endemic profit motive
43/
- Over-sensitive testing methodology
- Major logistics constraints
- Opaque/shifting regulatory reqs
- Inexperienced vendors and stakeholders
- Endemic profit motive
43/

 Read on Twitter
Read on Twitter