1/
Ondansetron in pregnancy revisited: We challenge the EMA SmPC pregnancy labelling:
@MotherToBaby @medsinpregnancy @WRISK_project #MedTwitter @Epi_D_Nique @MarleenvGelder @EMA_News #medsinpregnancy @yckaplan
HT @clearyb1 @ken_hodson https://onlinelibrary.wiley.com/doi/10.1111/bcpt.13541
Ondansetron in pregnancy revisited: We challenge the EMA SmPC pregnancy labelling:
@MotherToBaby @medsinpregnancy @WRISK_project #MedTwitter @Epi_D_Nique @MarleenvGelder @EMA_News #medsinpregnancy @yckaplan
HT @clearyb1 @ken_hodson https://onlinelibrary.wiley.com/doi/10.1111/bcpt.13541
2/
In 2019 EMA changed pregnancy labelling for ondansetron to "should not be used in the first trimester of pregnancy"
This effectively put patients and physicians between a rock and a hard place.
Decision based on 2 large epidemiological studies each comprising > 80.000 exposed
In 2019 EMA changed pregnancy labelling for ondansetron to "should not be used in the first trimester of pregnancy"
This effectively put patients and physicians between a rock and a hard place.
Decision based on 2 large epidemiological studies each comprising > 80.000 exposed
3/
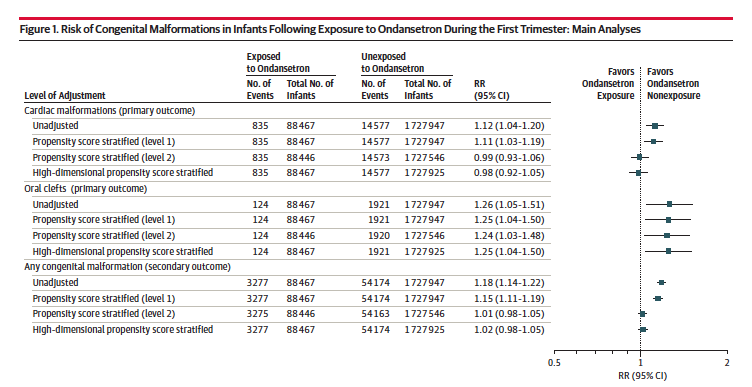
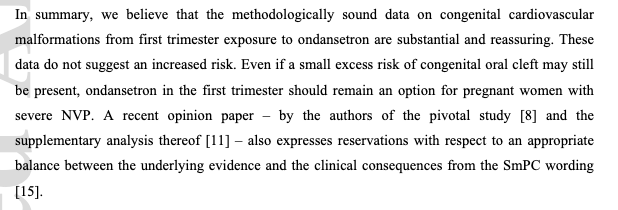
One methodologically sound study found no increased overall risk of congenital malformations or cardiac malformations and small increased risk for oral clefts (increasing the number from from 11 to 13 for every 1000 exposed liveborn).
https://pubmed.ncbi.nlm.nih.gov/30561479/
One methodologically sound study found no increased overall risk of congenital malformations or cardiac malformations and small increased risk for oral clefts (increasing the number from from 11 to 13 for every 1000 exposed liveborn).
https://pubmed.ncbi.nlm.nih.gov/30561479/
4/
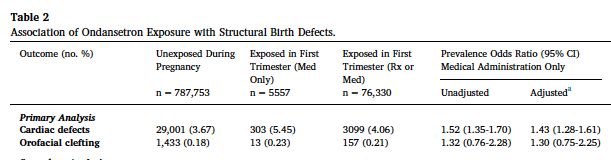
The other study reported a 43% increased risk of congenital cardiac malformations among those receiving a single-i.v. dose of ondansetron. This study is severely methodologically compromised
https://pubmed.ncbi.nlm.nih.gov/30385129/
The other study reported a 43% increased risk of congenital cardiac malformations among those receiving a single-i.v. dose of ondansetron. This study is severely methodologically compromised
https://pubmed.ncbi.nlm.nih.gov/30385129/
5/
a) Severe selection bias
b) Lack of external validity (extremely high rate, 3.7% of cardiac malformations among unexposed)
c) The authors did not respond to 5 specific supplementary questions requested for clarification from the EMA
a) Severe selection bias
b) Lack of external validity (extremely high rate, 3.7% of cardiac malformations among unexposed)
c) The authors did not respond to 5 specific supplementary questions requested for clarification from the EMA
6/
d) Severe COI - initially not fully disclosed:
I: Initial COI declared: "As an organization, TTi reports receiving funds from plaintiff law firms involved in ondansetron litigation...”
II: In fact, this study received substantial funding ($ 210,000) from..
d) Severe COI - initially not fully disclosed:
I: Initial COI declared: "As an organization, TTi reports receiving funds from plaintiff law firms involved in ondansetron litigation...”
II: In fact, this study received substantial funding ($ 210,000) from..
7/
II:..plaintiff’s representation who were pursuing litigative damage by claiming malformations from ondansetron use in pregnancy


https://www.govinfo.gov/content/pkg/USCOURTS-mad-1_15-md-02657/pdf/USCOURTS-mad-1_15-md-02657-16.pdf
II:..plaintiff’s representation who were pursuing litigative damage by claiming malformations from ondansetron use in pregnancy



https://www.govinfo.gov/content/pkg/USCOURTS-mad-1_15-md-02657/pdf/USCOURTS-mad-1_15-md-02657-16.pdf
8/
We - The Scientific Committee (less one recused member) of European Network of Teratology Informations Services -ENTIS- argue:
a) The Zambelli-Weiner paper is severely flawed and cannot carry any weight in the totality of evidence on the safety of ondansetron in pregnancy
We - The Scientific Committee (less one recused member) of European Network of Teratology Informations Services -ENTIS- argue:
a) The Zambelli-Weiner paper is severely flawed and cannot carry any weight in the totality of evidence on the safety of ondansetron in pregnancy
9/
b) The totality of the evidence on the safety of ondansetron in pregnancy is strong:
I: No overall increased risk of major malformations
II: No increased risk of cardiac malformations
III: A small excess risk of oral cleft
b) The totality of the evidence on the safety of ondansetron in pregnancy is strong:
I: No overall increased risk of major malformations
II: No increased risk of cardiac malformations
III: A small excess risk of oral cleft
10/
c) The SmPC designation "ondansetron should not be used in the first trimester of pregnancy" is
I: Not sufficiently supported by evidence and therefore
II: Not justifiable
III: Not in the interest of pregnant women suffering from severe NVP
c) The SmPC designation "ondansetron should not be used in the first trimester of pregnancy" is
I: Not sufficiently supported by evidence and therefore
II: Not justifiable
III: Not in the interest of pregnant women suffering from severe NVP
11/
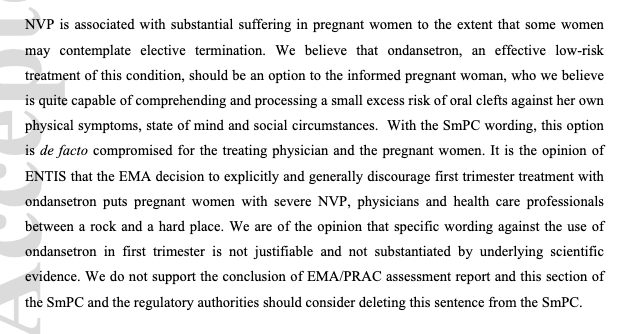
c)
IV:We believe these women are quite capable of comprehending and processing a small excess risk of oral cleft and making an informed decision
V: We suggest this SmPC section be reevaluated
---end---
c)
IV:We believe these women are quite capable of comprehending and processing a small excess risk of oral cleft and making an informed decision
V: We suggest this SmPC section be reevaluated
---end---

 Read on Twitter
Read on Twitter