with Scotland due to start vaccination next week with the Pfizer/BioNTech BNT162b2 coronavirus vaccine, what do we know about it?
a thread
https://www.bbc.co.uk/news/uk-scotland-54984390
a thread
https://www.bbc.co.uk/news/uk-scotland-54984390
first of all, what type of vaccine is it?
BNT162b2 is an mRNA vaccine. Although the SARS-CoV-2 vaccines are the first of this family to be licensed, they have been studied for many years in influenza, Zika, rabies and RSV @CDCgov
https://www.cdc.gov/vaccines/covid-19/hcp/mrna-vaccine-basics.html
BNT162b2 is an mRNA vaccine. Although the SARS-CoV-2 vaccines are the first of this family to be licensed, they have been studied for many years in influenza, Zika, rabies and RSV @CDCgov
https://www.cdc.gov/vaccines/covid-19/hcp/mrna-vaccine-basics.html
the mRNA in the vaccine contains nucleoside modifications to make it more stable, and is packaged in lipid nanoparticles so that the the mRNA is taken up by cells.
as mRNA is the minimal genetic vector, anti-vector immunity is avoided.
https://www.nature.com/articles/nrd.2017.243
as mRNA is the minimal genetic vector, anti-vector immunity is avoided.
https://www.nature.com/articles/nrd.2017.243
the mRNA is for a full length spike glycoprotein
the SARS-CoV-2 spike protein transcript has been modified by two proline mutations to lock it in the prefusion conformation and make it more immunogenic
https://pubmed.ncbi.nlm.nih.gov/32075877/
https://pubmed.ncbi.nlm.nih.gov/28807998/
the SARS-CoV-2 spike protein transcript has been modified by two proline mutations to lock it in the prefusion conformation and make it more immunogenic
https://pubmed.ncbi.nlm.nih.gov/32075877/
https://pubmed.ncbi.nlm.nih.gov/28807998/
The idea is that the lipid nanoparticles fuse with host cells, which releases the mRNA into these cells. The mRNA doesn't enter the nucleus or integrate into the genome of host cells
The transcript is then translated by the cells, and undergoes post-translational modification
The transcript is then translated by the cells, and undergoes post-translational modification
to produce a modified spike protein that is expressed on the cell surface and produces an immune response
ideally, this protein then elicits both humoral and cytotoxic T cell responses
ideally, this protein then elicits both humoral and cytotoxic T cell responses
what has been published on the effects of BNT162b2? A Phase I study published this October @NEJM looked at the effects of injecting the vaccine into healthy volunteers
https://www.nejm.org/doi/full/10.1056/NEJMoa2027906
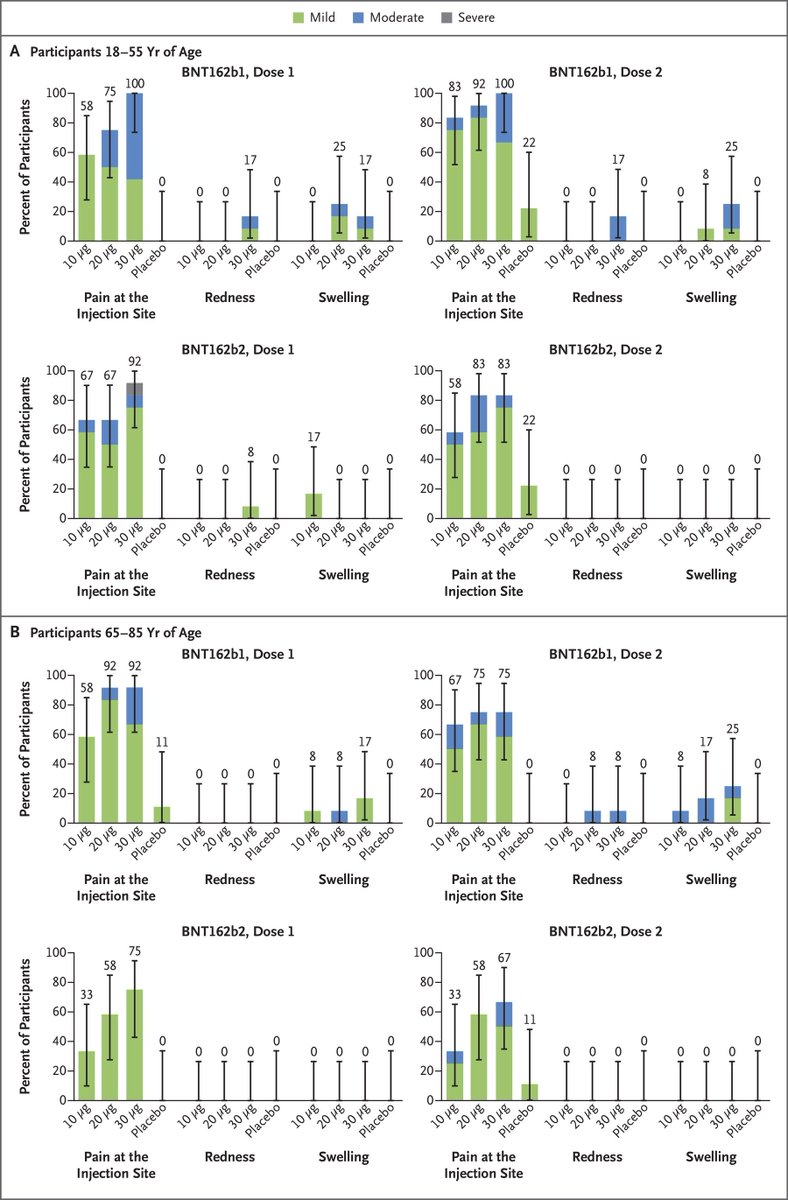
72 participants 18 to 85 years received BNT162b2 & 18 volunteers placebo
https://www.nejm.org/doi/full/10.1056/NEJMoa2027906
72 participants 18 to 85 years received BNT162b2 & 18 volunteers placebo
a reasonable number of these volunteers reported pain at the vaccination site; a much smaller number reported redness or swelling
there were no Grade 4 local reactions
there were no Grade 4 local reactions
Systemic events (fatigue, headache, chills, muscle pain, and joint pain) were reported in small numbers of younger recipients of BNT162b2
there were no grade 4 systemic events
there were no grade 4 systemic events
The investigators examined antibody responses to SARS-CoV-2, compared to a convalescent serum panel. For those in 18-55 age group, mean geometric titres for neutralisation were between 1.7 to 4.6 times the convalescent serum panel; and 1.1 to 2.2 times for those aged 65 to 85
What about the balance between TH1 and TH2 responses?
the authors state that "cell-mediated immune responses (Th1-biased CD4+ and CD8+) elicited by BNT162b1 have been observed and reported in the German trial ( https://pubmed.ncbi.nlm.nih.gov/32998157/ ) ...
the authors state that "cell-mediated immune responses (Th1-biased CD4+ and CD8+) elicited by BNT162b1 have been observed and reported in the German trial ( https://pubmed.ncbi.nlm.nih.gov/32998157/ ) ...
the cellular immune responses elicited by BNT162b2 are still being studied"
this is an important consideration when thinking about the risk of VAERD https://pubmed.ncbi.nlm.nih.gov/32385100/
this is an important consideration when thinking about the risk of VAERD https://pubmed.ncbi.nlm.nih.gov/32385100/
What about the vaccine's performance in Phase II/III? Firstly, the press release from Pfizer. The dose chosen for the trial was the highest one from the NEJM paper: 30 µg, and the dosing also the same- 2 injections given 21 days apart as a IM injection
https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-conclude-phase-3-study-covid-19-vaccine
https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-conclude-phase-3-study-covid-19-vaccine
Their study enrolled 43,661 participants, with vaccine and placebo distributed equally. For primary efficacy analysis 36,621 participants without prior evidence of infection were analysed, 18,242 in the vaccine & 18,379 in the placebo group
Details here
https://www.gov.uk/government/news/uk-authorises-pfizer-biontech-covid-19-vaccine
Details here
https://www.gov.uk/government/news/uk-authorises-pfizer-biontech-covid-19-vaccine
Pfizer/BioNTech evaluated 170 cases of COVID-19 in trial participants. Of these cases, 162 occurred in the placebo group, and 8 in the BNT162b group.
This gives a efficacy of 95% (credible interval of 90.3% to 97.6%)
This gives a efficacy of 95% (credible interval of 90.3% to 97.6%)
How was the trial conducted? This is described here https://clinicaltrials.gov/ct2/show/NCT04368728
The primary outcomes of interest were:
-Confirmed COVID-19 in Phase 2/3 participants
-Confirmed severe COVID-19 in Phase 2/3 participants
The primary outcomes of interest were:
-Confirmed COVID-19 in Phase 2/3 participants
-Confirmed severe COVID-19 in Phase 2/3 participants
coronavirus disease was defined on basis of a positive RT-PCR result and 1 or more symptom consistent with COVID-19 disease:
fever, new cough, shortness of breath, chills, muscle pain, loss of sense of taste/smell, sore throat, diarrhoea or vomiting
fever, new cough, shortness of breath, chills, muscle pain, loss of sense of taste/smell, sore throat, diarrhoea or vomiting
What we don't know is whether the vaccine prevented asymptomatic disease- details for these aspects of the trial are currently unavailable
In summary
-No evidence to date of adverse effects specific to mRNA vaccines
-No evidence to date of adverse effects specific to the BNT162b2 vaccine
-BNT162b2 appears to be highly effective, but no data yet on asymptomatic infection/transmission https://www.bbc.co.uk/news/uk-scotland-55156783
-No evidence to date of adverse effects specific to mRNA vaccines
-No evidence to date of adverse effects specific to the BNT162b2 vaccine
-BNT162b2 appears to be highly effective, but no data yet on asymptomatic infection/transmission https://www.bbc.co.uk/news/uk-scotland-55156783

 Read on Twitter
Read on Twitter