Latest work from the lab!
Identity matters:
Lethal high-grade glioma arises from misregulation of interneuron differentiation
Big team effort with Nada Jabado,
puzzle solved by @CarolCLChen @ShriyaDeshmukh3 @selinjessa @DjidjiJdaJdah
A thread https://twitter.com/CellCellPress/status/1333433958483300353
https://twitter.com/CellCellPress/status/1333433958483300353
Identity matters:
Lethal high-grade glioma arises from misregulation of interneuron differentiation
Big team effort with Nada Jabado,
puzzle solved by @CarolCLChen @ShriyaDeshmukh3 @selinjessa @DjidjiJdaJdah
A thread
 https://twitter.com/CellCellPress/status/1333433958483300353
https://twitter.com/CellCellPress/status/1333433958483300353
Brain tumors have exquisite regional and temporal specificity. The reasons are not always clear.
What makes certain cells permissive to damaging mutations in essential genes, such as histones?
2/7
What makes certain cells permissive to damaging mutations in essential genes, such as histones?
2/7
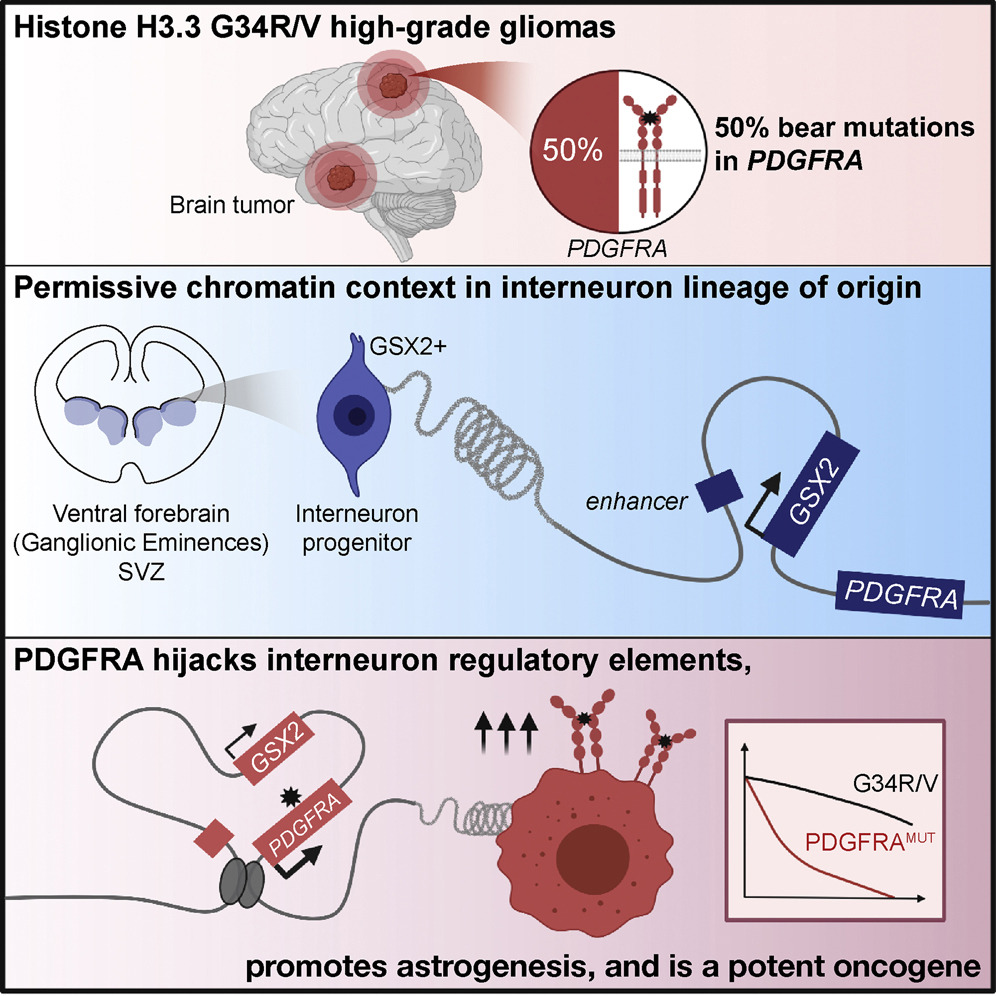
With this question in mind, we tackled histone H3.3 G34-mutant high-grade #gliomas.
They have complex histopathology, with both neuronal and glial components. Which one reflects their true identity?
(art by @selinjessa )
3/7
They have complex histopathology, with both neuronal and glial components. Which one reflects their true identity?
(art by @selinjessa )
3/7
We looked for answers in genomic, epigenomic and transcriptomic tumor data (n=95).
First, @CarolCLChen discovered a specific, high frequency of activating PDGFRA mutations…
Mutant PDGFRA is a potent oncogene on its own.
4/7
First, @CarolCLChen discovered a specific, high frequency of activating PDGFRA mutations…
Mutant PDGFRA is a potent oncogene on its own.
4/7
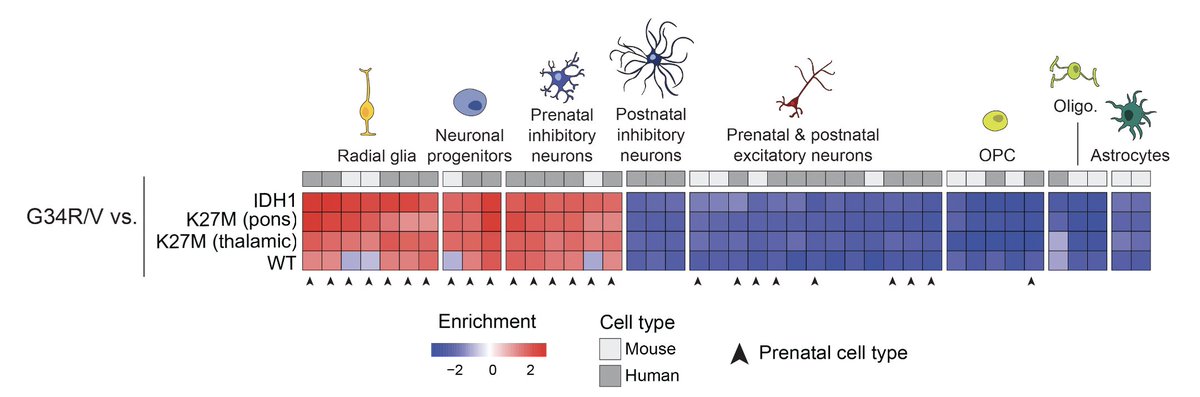
Next, using a single-cell atlas of the developing brain, @selinjessa found that G34R/V tumors map to Gsx2+ interneuron progenitors from the prenatal brain and the subventricular zone.
But why PDGFRA mutations are specific to Gsx2+ progenitors?
5/7
But why PDGFRA mutations are specific to Gsx2+ progenitors?
5/7
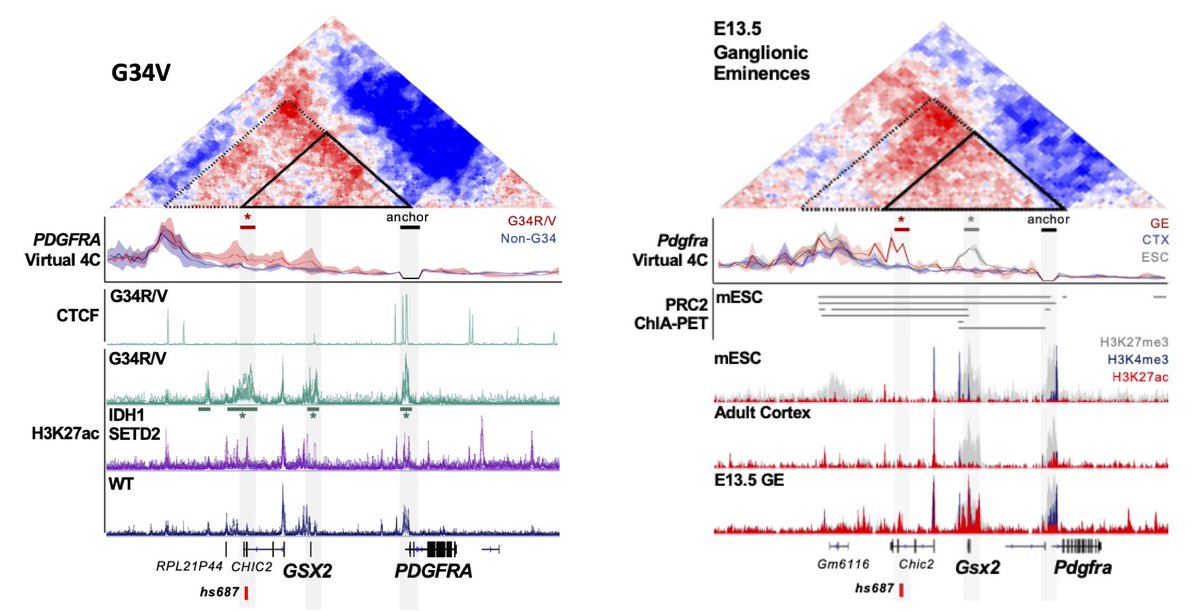
The #3Dgenome gave us the answer:
An interaction loop links PDGFRA with the active GSX2 regulatory elements in tumor cells.
Also formed in ganglionic eminences, where interneurons are born...
@DjidjiJdaJdah @CarolCLChen @ShriyaDeshmukh3
6/7
An interaction loop links PDGFRA with the active GSX2 regulatory elements in tumor cells.
Also formed in ganglionic eminences, where interneurons are born...
@DjidjiJdaJdah @CarolCLChen @ShriyaDeshmukh3
6/7
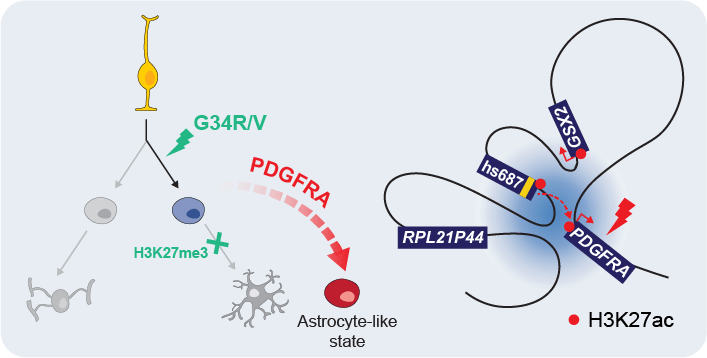
Our model: G34R/V tumors have an interneuron origin, and chromatin context in this cell of origin facilitates PDGFRA co-option for oncogenesis
Extremely thankful for the work of Paolo Salomoni, @dtwjones and all other authors. A true team effort.
7/7
Extremely thankful for the work of Paolo Salomoni, @dtwjones and all other authors. A true team effort.
7/7

 Read on Twitter
Read on Twitter