trying my hands at @OnlyNakedTruth 'esque thread. /0
Everyone knows energy is the basis of life. Energy as in the physical definition of energy [F x Dist kind of energy] and not the cosmic kind of energy. I don't know about the latter for writing a tweet - let alone a thread.
Life is diverse. You find life in sub-oceanic volcanic vents, Alkaline lakes [btw, it is far more difficult for life to exist in alkalinity than in acidity. Story for another time], acid lakes, artcic, antarctic, in Mariana trench, & quite certainly on Sagarmata too.
Life exhibits unimaginable variety in shapes and sizes. Despite such bewildering diversity, life uses only one currency for energy. An innocuously named molecule called "Adenosine Triphosphate" or more famously, ATP.
one fundamental question is, obviously, how all five branches of life - archae, fungi, algae, plantae, and animalia have evolved the biochemical means to produce and consume the same molecule for energy. Suffices to say: both proton powered and electron powered stuff produces ATP
Two more fundamental questions remain. One is more popular and hence more people know the answer: why "triphosphate"? or more generally, the question is posed as "why pyrophosphate"! .. however the second and more intriguing question is often given a pass: why adenosine?
To understand why this question is such a fundamental conundrum - we have to dig a bit into biochemistry / chemistry. DNA has four bases: Adenosine [yes, the same fellow], Guanosine [cousin of our dear adenosine], Cytosine [partner of Guanosine] and Thymine [partner of Adenosine]
The first letters of these fellows make up the famous ATGC of DNA / genetic code. These are carbon-nitrogen ring molecules, that form the steps of the DNA ladder (in pairs, of course).
pertinent point: all of these molecules are "activated" by addition of sugar & phosphate ion.
pertinent point: all of these molecules are "activated" by addition of sugar & phosphate ion.
Usually, a fully activated base has a chain of three phosphate ions [hence the name "tri" phosphate] attached to it. The energy carried by the tri-phosphate ion is not affected by the base to which it is attached (technically, the nucleoside). So, irrespective of whether ++
++ you have ATP, GTP, CTP, or TTP, the energy that a biological system can derive from it, remains the same. In fact, in modern biological systems, ATP is used as general currency for energy, GTP is used to power protein synthesis, CTP for lipid synthesis & UTP (similar to TTP)+
++ is used in the activation of complex sugars. They have equivalent energy and are known to swap their phosphate ions to transfer energy from one fellow to another.
So - for all practical purposes - the energy from all these sources is the same.
So - for all practical purposes - the energy from all these sources is the same.
To give an analogy - imagine you have a thermal power plant, a hydel plant, and a nuclear power plant :: technically, your electric fan shouldn't care about where the electricity comes from - right? Apparently, over the entirety of life, the cells care. Weird, isn't it?
Then - the fundamental question before us presents itself. Why is that ATP is the currency of energy across all life forms but not any other. Remember - when life arose in its basic modern form [info stored in nucleotides] about 3 billion years ago, all four bases existed.
It couldn't be convergent evolution - where different streams of life picked the same molecule *by chance*, amongst other choices. If it were so, we would have seen at least some major groups where this rule isn't true. The lack of exceptions till date rules conv. evol. out.
If we rule out Convergent Evolution as an explanation, it leaves two other possibilities: 1) all life arose at one point, and that the first-ever life form somehow used ATP, and then in true life fashion of "don't fix it if it ain't broken", everyone did the same for 3B yrs. or +
+ 2) there was some sort of prior condition that made ATP or one of its precursors the most obvious [higher presence] choice!
While both look likely given the "primordian soup theory" -- where apparently life arose in hot boiling chemical oceans .. recent research suggests ++
While both look likely given the "primordian soup theory" -- where apparently life arose in hot boiling chemical oceans .. recent research suggests ++
++ life actually arose in isolated small ponds. Given the innumerable number of hot and deadly ponds that were available in the primordial earth, if there were all options available, convergent evolution would have been ruled out as a major factor. That leaves us with option (2).
That is - somehow, amongst all possible choices, Adenosine (A) was the most present / highly available molecule, and those that evolved to use it had the highest chance of success.
And that seems to be the right answer so far. But, then it doesn't still answer "why Adenosine?".
And that seems to be the right answer so far. But, then it doesn't still answer "why Adenosine?".
To answer that question, we need to go a little bit backwards in time - from 3B years to about 3.2-3.5B yrs ago. What the earth had then, is known as a "reducing atmosphere". We had lot of gases none of which would support modern life. Oxygen was absent. We had poisonous gases.
Around 1950, two scientists - Urey and Miller - performed a nifty experiment. They showed that these gases + water in the presence of large amounts of energy [lighting in this case] could form basic building block molecules -- like Amino Acids and formaldehyde.
A big criticism of Urey and Miller's experiment until recently was that they didn't find A/ T/ G or C: the information molecules. However, this conundrum was recently solved in 2017 - where scientists (Ferus, Pietrucci et al, 2017) showed evidence of showing formaldehyde ++
++ can be used to synthesis A / T / C / G. This resolves one part of the puzzle.
The other part is still why "A", but not C/G/T?
[As an aside, that Urey and Miller didn't find A/T/G/C was a big objection to evolution by creationists; that problem rests now]
The other part is still why "A", but not C/G/T?
[As an aside, that Urey and Miller didn't find A/T/G/C was a big objection to evolution by creationists; that problem rests now]
// I promise, we are near the end //
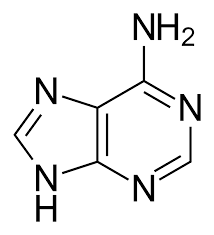
We take a look at the chemical formula for Adenine -- it is C5-N5-H5 .. doing a bit of logical jugglery, we can get (CNH)x5 for the empirical formula.
Rearrange CNH, and we actually get H-CN (HCN), which is a primodrial earth gas.
We take a look at the chemical formula for Adenine -- it is C5-N5-H5 .. doing a bit of logical jugglery, we can get (CNH)x5 for the empirical formula.
Rearrange CNH, and we actually get H-CN (HCN), which is a primodrial earth gas.
While it is easy to logically suggest that HCN forms adenine and that the abundance of HCN made adenine abundant, based on stoichiometry, we have to prove that it is chemically possible to do this transformation.
Enter John Oro - 1961. In their lab, Oro and colleagues ++
Enter John Oro - 1961. In their lab, Oro and colleagues ++
++ proved that HCN at high temperatures can simply polymerize to form Adenine.
Now we can apply Occam's razor to the problem. One on hand, we have a fundamental molecule which can simply be obtained by baking a gas in high temps [the gas was ubiquitous as were high temps]. ++
Now we can apply Occam's razor to the problem. One on hand, we have a fundamental molecule which can simply be obtained by baking a gas in high temps [the gas was ubiquitous as were high temps]. ++
OTOH - we have other options that need more chemicals than just the HCN gas [for instance, one or more molecules of formaldehyde].
Clearly, the first choice was more likely. That is why scientists think ATP became the energy currency of the cell - but not G/T/U/CTP. /Fin.
Clearly, the first choice was more likely. That is why scientists think ATP became the energy currency of the cell - but not G/T/U/CTP. /Fin.

 Read on Twitter
Read on Twitter