A thread on vaccine safety, adverse events following immunization (AEFI) and assessments that happen when vaccines are tested before licensure. WHO has a great course at https://vaccine-safety-training.org/home.html from which a couple of figures are taken. 1/21
No vaccine (or drug from aspirin to zinc) is absolutely risk free. BUT no vaccine is licensed where benefits don't hugely outweigh any risk. To know risk is rare is no consolation when AEFIs occur, but important as a society to understand risks exist & are measured. 2/21
Most common adverse events following vaccination are minor (pain, redness, swelling), but occasionally rare and serious adverse events (Seizures, anaphylaxis or severe allergic reactions) can happen. 3/21
We have a low tolerance of severe adverse events because vaccines are given to healthy people. The bar for safety is high. Vaccine trials include more people at each stage so that safety is tracked in increasing numbers. 4/21
Therefore, Phase I trials for safety in 10-100 people. Phase II trials in 100-1000 people for safety/ immunogenicity. Phase III trials in 1000-10000 or more for safety/efficacy. After vaccines are licensed, safety surveillance continues in phase IV or programmatic use. 5/21
But today, focus is on pre-licensure assessment. What happens if a study participant becomes seriously ill? First, the participant must be looked after and provided all needed care. 6/21
Then the Principal Investigator at the site must report all that is known about participant and his/her illness to the institutional ethics committee (IEC), the sponsor (usually the company making the vaccine) and the regulator (Drugs Controller General of India). 7/21
The investigator offers his/her opinion regarding whether the illness is related to vaccination, but that opinion and all data is sent to the sponsor. The sponsor will inform the independent Data Safety Monitoring Board (DSMB). 8/21
The DSMB usually has independent experts (clinical trialist, disease specialist, trial statistician) who will review the data in detail and make a report that the sponsor has to share with trial site IEC and the regulator. 9/21
Adverse events following immunization are of five types. 1. Vaccine product related reaction. e.g. intussusception (telescoping of the intestine) seen in the 1st wk after rotavirus vaccination, happens in some places (not all) in 1 in 16,000 or 1 in 60,000 vaccinated kids. 10/21
2. Vaccine quality defect related reaction. E.g. inactivated polio vaccine was not completely inactivated and caused polio (Read about the Cutter Incident) 3. Immunization error related reaction e.g. wrong diluting fluid used and children become paralysed (happened). 11/21
4. Immunization anxiety related reaction. e.g. school based immunization where a child is scared and faints
5. Coincidental event: An AEFI that is not due to any of the above 4. E.g. a child develops fever after vaccination, but it is dengue and not vaccine related. 12/21
5. Coincidental event: An AEFI that is not due to any of the above 4. E.g. a child develops fever after vaccination, but it is dengue and not vaccine related. 12/21
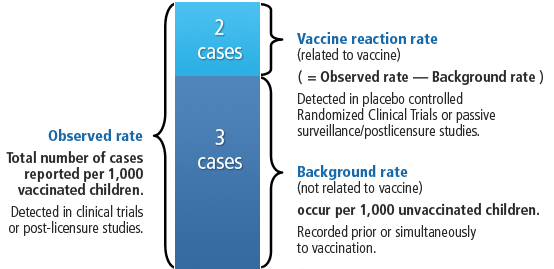
Vaccine adverse events occur with a certain frequency, so knowing the background rates is important, so that we can figure out what is due to the vaccine and what would happen if there was no vaccine. See figure. 13/21
Adverse events are classified as very common (more than 10%), common (1 to 10%), uncommon (0.1 to 1%), rare (1 in 10,000), very rare (1 in 100,000 or less). 14/21
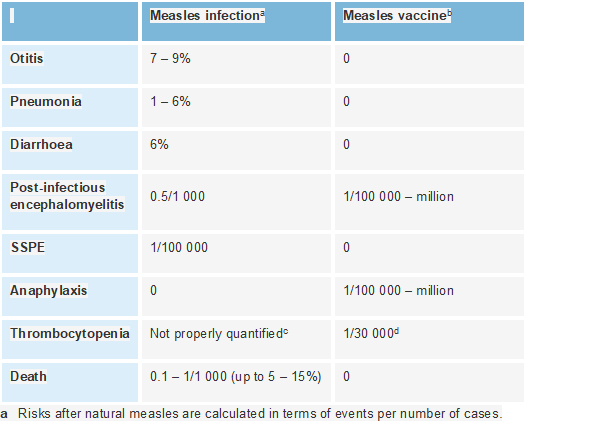
Measles vaccine has very rare serious adverse events but risk benefit analysis is heavily weighted towards vaccination, because of serious consequences of infection. 15/21
But when thousands of people get vaccines in trials, some rare events are likely to only be picked up in phase III or later. But also some illnesses are bound to happen to vaccinated people even if the vaccine is safe. How to decide what is what? 16/21
In other words, when there is a serious adverse event, it is important to determine its relationship to the vaccination. Was the event definitely, probably, possibly caused by the vaccine? Was it definitely not caused by the vaccine? 17/21
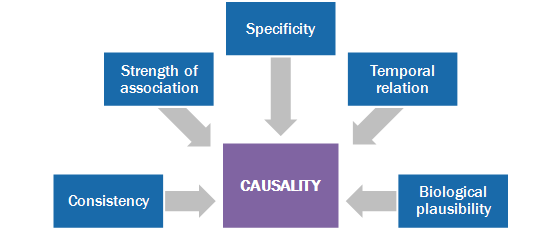
Vaccine safety experts use an approach called causality assessment, which is based on 5 principles. This needs expertise. 18/21
And even then, an absolute determination may not be possible. But if an association cannot be ruled out, then the researchers, sponsor, DSMB will take extra care to follow up on the AEFI that might be an early safety signal. 19/21
Every new treatment or prevention requires experimentation & carries risk. We must respect those who participate in clinical trials as volunteers for a greater good. And as clinical researchers, our responsibility to ensure that all that we can do to minimise risk is done. 20/21
But if a participant becomes ill, especially in a vaccine trial, all medical and psychological support possible has to be provided as we do our best to assess relatedness. That is a individual and collective responsibility. 21/21

 Read on Twitter
Read on Twitter