#OncoAlert Available @NEJM, results from the phase III CROWN trial of lorlatinib versus crizotinib as first line treatment for #ALK NSCLC. Initially presented at virtual #ESMO20, lorlatinib had a significantly better PFS (HR 0.28) #LCSM https://www.nejm.org/doi/full/10.1056/NEJMoa2027187?query=featured_home
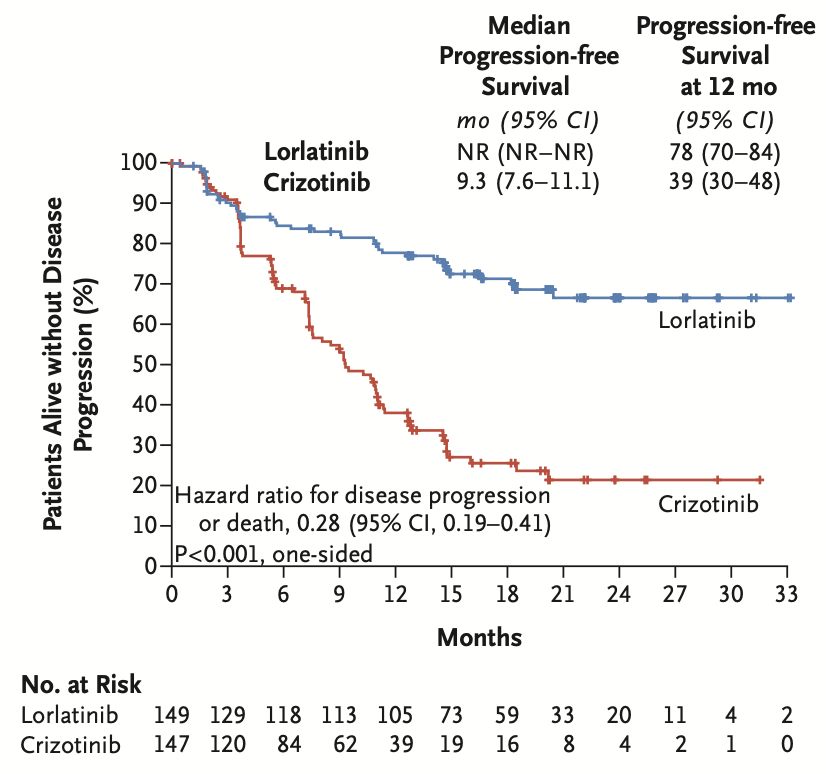
296 patients with treatment-naive ALK+ NSCLC (by IHC) were randomized to lorlatinib 100mg qd or crizotinib 250mg bid. Lorlatinib had higher RR (76% vs 58%) and significantly superior PFS (median not reached, HR 0.28, 1y PFS rate 78% vs 39%). #LCSM
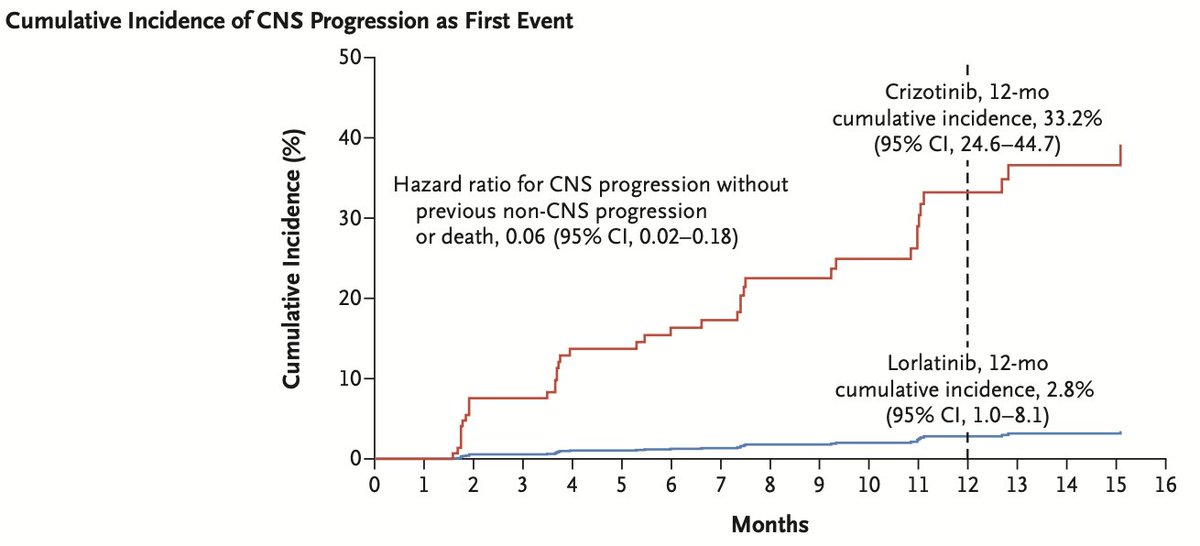
Lorlatinib has exceptional CNS penetration and CNS RR favored lorlatinib (66% vs 20%). CNS progression (as first event) was uncommon in the lorlatinib arm (12m incidence was only 2.8% versus 33.2% with crizotinib). Too early for OS. #LCSM
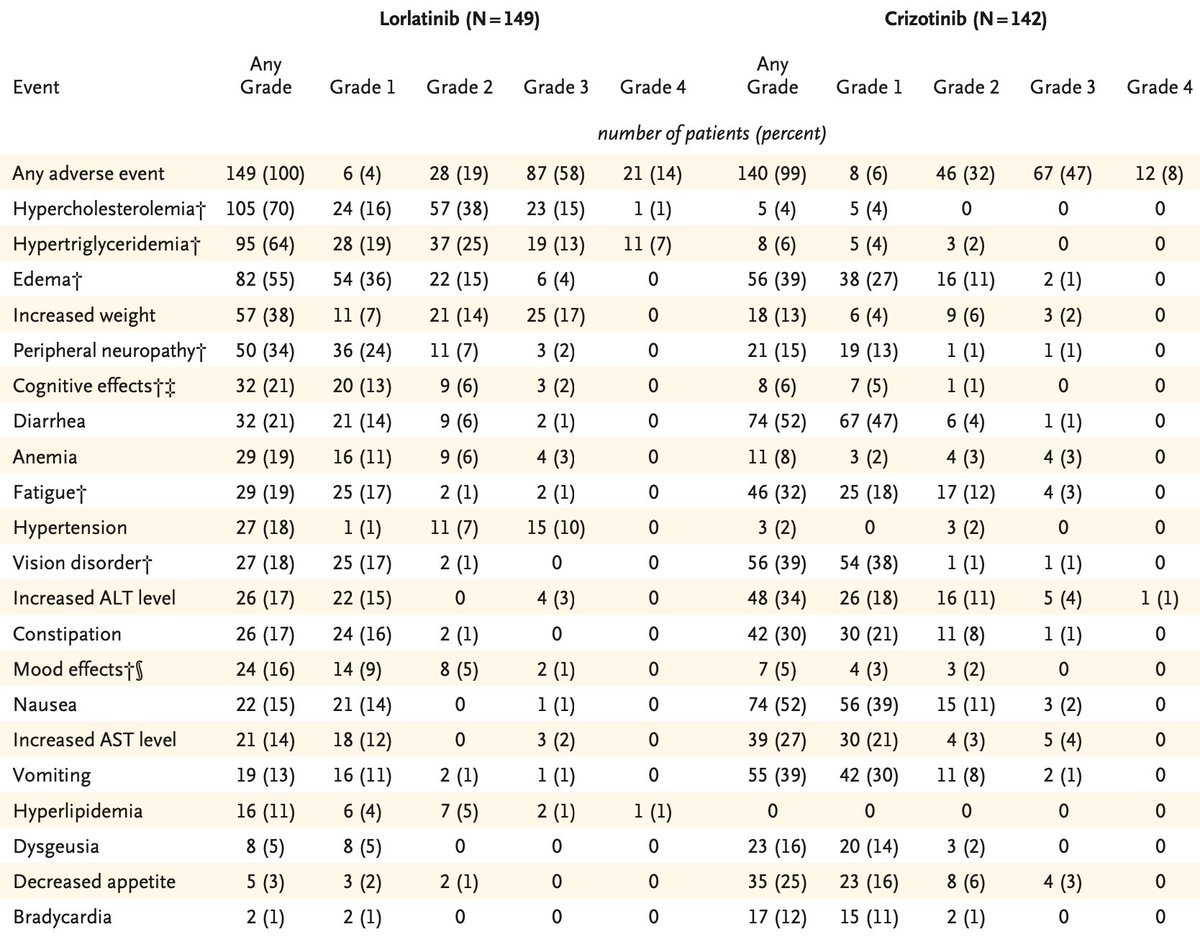
Must note unique toxicities with lorlatinib including hypercholesterolemia (70%), weight gain (38%), peripheral neuropathy (34%), HTN (18%). Most worrisome are the cognitive effects (21%) which include memory, attention, mood - these can be subtle and possibly severe.
Overall, CROWN was markedly positive and likely to lead to lorlatinib approval. Exciting #ALK option but patients and prescribers need to be aware of the cognitive effects - improve with dose reduction but early recognition is critical to avoid bad outcomes. Great team involved!

 Read on Twitter
Read on Twitter