We are excited to publish our paper entitled “Female mice exposed to low-doses of dioxin during pregnancy and lactation have increased susceptibility to diet-induced obesity and diabetes” in #Molecular Metabolism! https://doi.org/10.1016/j.molmet.2020.101104 #Bruinlab (1/13)
Human studies consistently show an association between persistent organic pollutants, including dioxin (aka TCDD), and increased diabetes risk; however, a causal link has not been established. (2/13)
We assessed the short- and long-term impact of transient low-dose TCDD exposure during pregnancy/lactation on glucose metabolism and beta cell function in female mice. (3/13)
We stopped TCDD exposure at the end of lactation and monitored vehicle- and TCDD-exposed dams for up to 10 weeks on a chow diet. Next, dams were either transferred to high-fat diet (HFD) or remained on chow diet for another 11 weeks (i.e. a total of 21 weeks post-TCDD). (4/13)
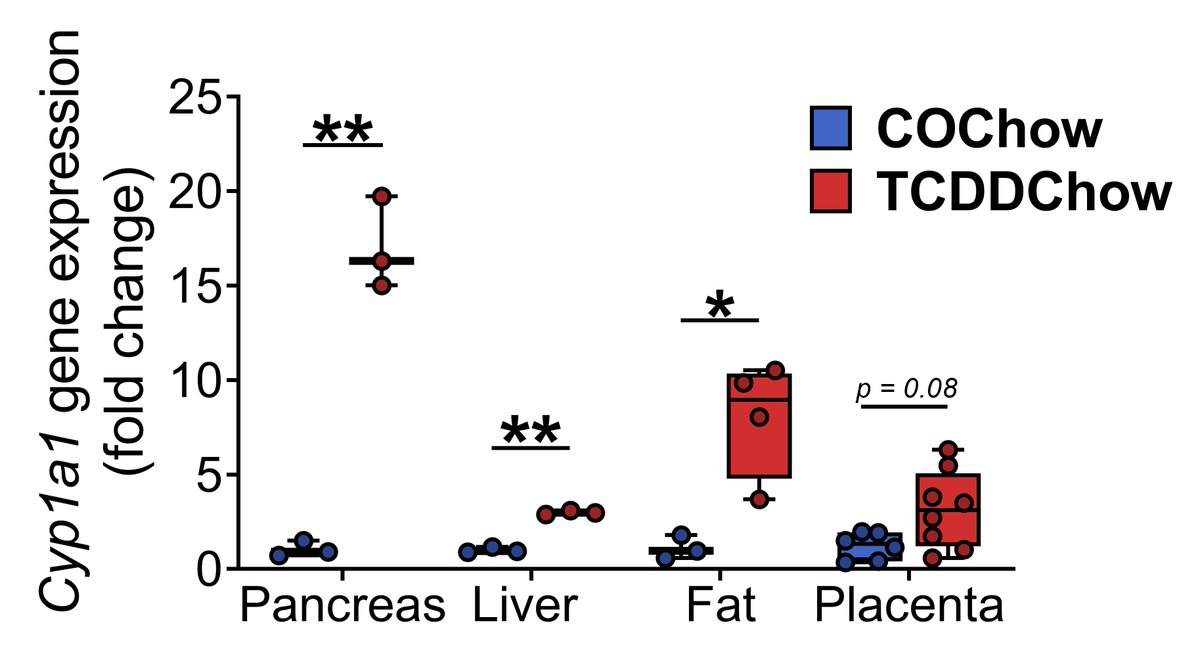
We used Cyp1a1 as a biomarker of direct cell/tissue exposure to TCDD (see DOI:10.1007/s00125-019-05035-0 http://doi.org/10.1038/s41598-020-57973-0 for more detail). Fig 1 shows that TCDD reaches the pancreas of pregnant dams and induces Cyp1a1 even more strongly than in the liver or fat. (5/13)
Stay tuned for work from our lab assessing whether Cyp1a1 induction in islets is protective or deleterious! (6/13)
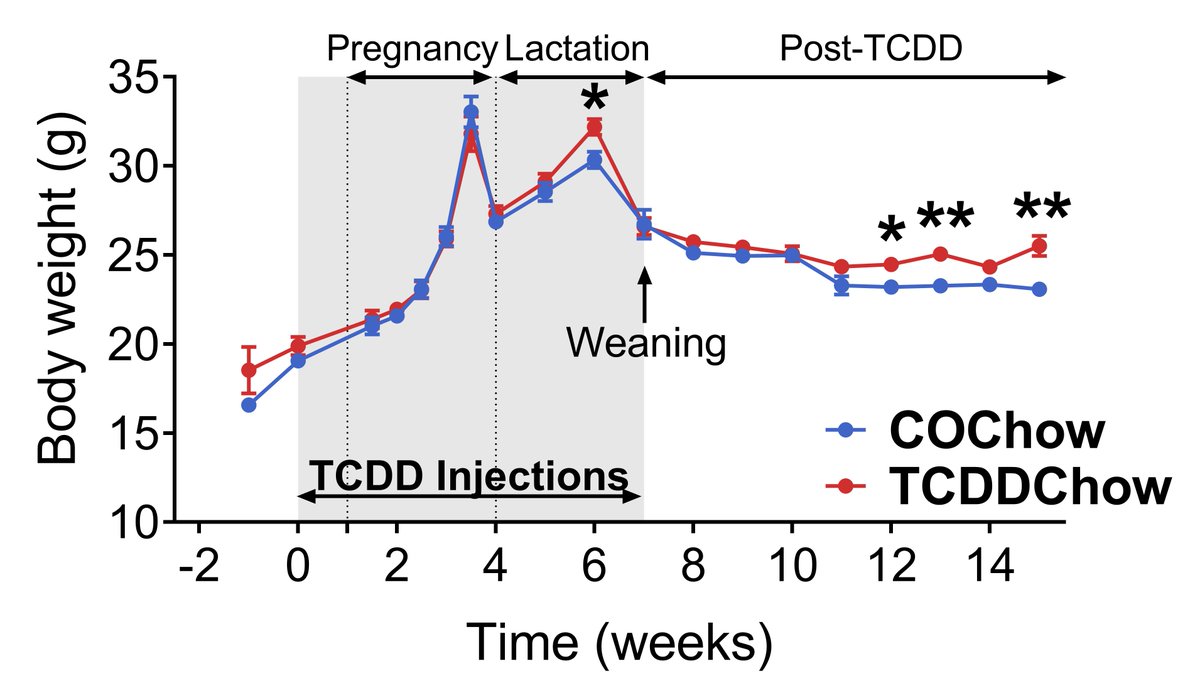
We found that TCDD exposure during pregnancy/lactation promoted weight gain in chow-fed dams long after TCDD was fully excreted. (7/13)
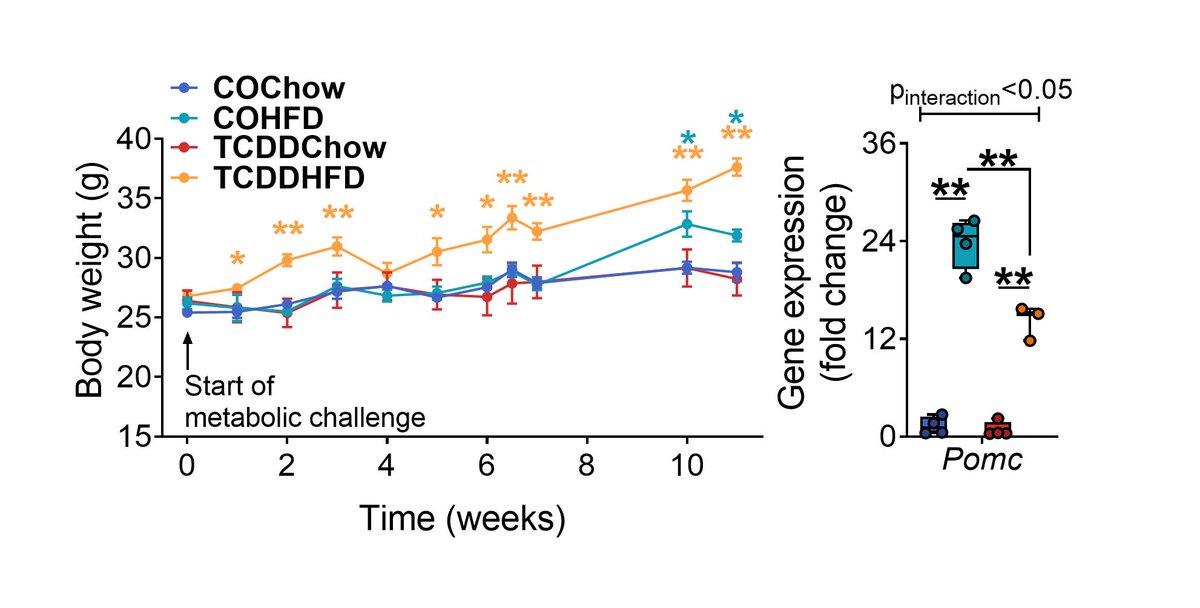
TCDD-exposed dams gained excessive weight compared to controls when transferred to a HFD later in life. This was associated with reduced hypothalamic expression of Pomc, a gene required to maintain energy homeostasis. Thank you @thecheelab for analysing the hypothalamus! (8/13)
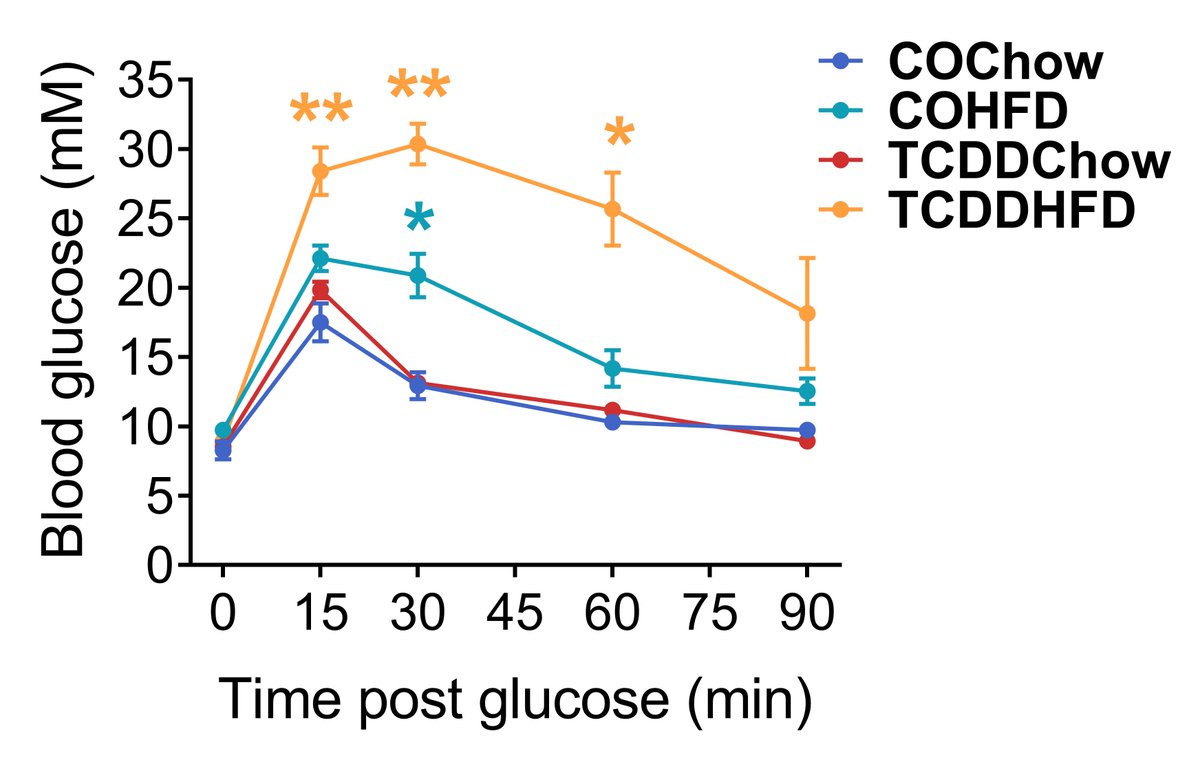
TCDD also accelerated the onset and amplified the degree of glucose intolerance in HFD-fed dams (Fig 4). This was associated with reduced glucose-induced plasma insulin levels (Fig 5). (9/13)
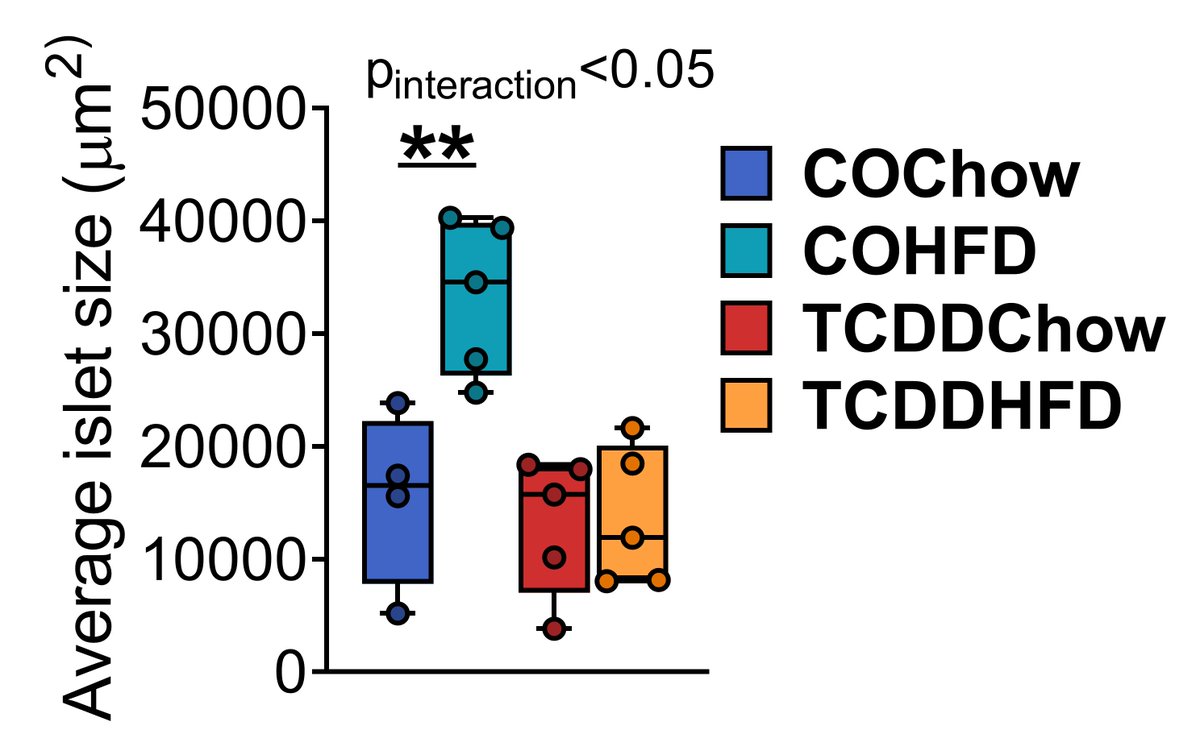
In Fig 6, we analysed islet morphology and composition to further investigate the phenotype observed in vivo. HFD-fed control dams had larger islets than chow-fed controls, which was expected. In contrast, TCDD-exposed dams lacked this important adaptation to HFD feeding. (10/13)
TCDDHFD dams also had a higher proportion of beta cells that lacked MAFA, a marker of beta cell maturity, and increased cytoplasmic proinsulin accumulation, suggestive of defects in proinsulin processing within beta cells (Fig 6). (11/13)
Our study suggests that transient low-dose TCDD exposure in female mice impairs metabolic adaptability to HFD feeding long after exposure ceases. Therefore, exposure to dioxin/dioxin-like pollutants may contribute to obesity and/or diabetes pathogenesis in females (12/13).
Thank you to everyone who contributed to this paper! @bruinjenny1 @thecheelab @MikaylaPayant @GeronimoMatteo (13/13)

 Read on Twitter
Read on Twitter