Super excited to share my first co-authored paper just out in @NatureChemistry!
We showed how chemical signaling between dissolving oil droplets can lead to non-reciprocal interactions resembling the chasing behavior between predator and prey: https://www.nature.com/articles/s41557-020-00575-0
We showed how chemical signaling between dissolving oil droplets can lead to non-reciprocal interactions resembling the chasing behavior between predator and prey: https://www.nature.com/articles/s41557-020-00575-0
This famous footage captured by Rogers in the 1950's shows the epic chase of a neutrophil hunting down a bacteria. No brains here, the behavior is mediated entirely by chemicals shared between the two.
But can we design synthetic systems and materials that can do the same?
But can we design synthetic systems and materials that can do the same?
Chasing requires the interaction between things be nonreciprocal (A attracted to B, B repelled by A).
However most physical interactions are reciprocal (things either attract or repel). Nature's trick is to operate outside equilibrium where such reciprocity is not guaranteed.
However most physical interactions are reciprocal (things either attract or repel). Nature's trick is to operate outside equilibrium where such reciprocity is not guaranteed.
To synthesize nonreciprocal interactions we used an emulsion system of microscale oil droplets dispersed in an aqueous surfactant solution.
The surfactant causes droplets to slowly dissolve, providing a sustained release of chemical energy to keep things out of equilibrium.
The surfactant causes droplets to slowly dissolve, providing a sustained release of chemical energy to keep things out of equilibrium.
Dissolving droplets have previously been shown to move in surfactant solutions due the way that gradients of dissolved oil affect the surfactant's ability to stabilize the droplet interface leading to differences in surface tension across drops.
OK, now let's cut to the chase...
OK, now let's cut to the chase...
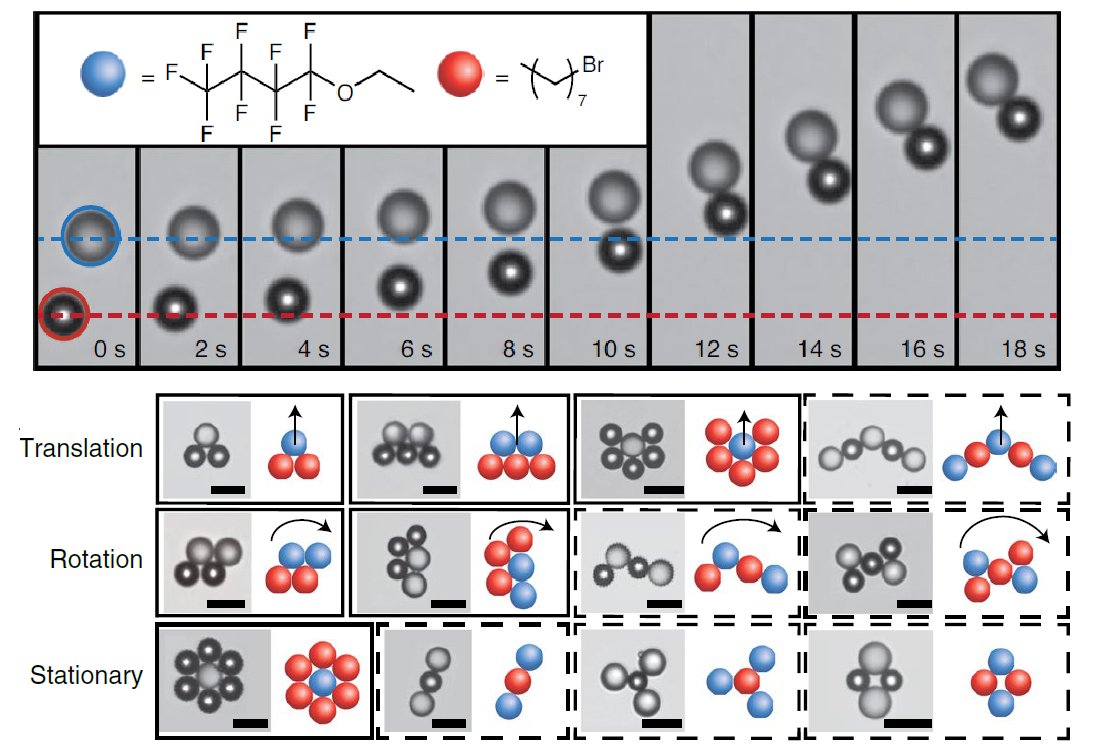
Picking two oils which were chemically distinct, having different interactions with the surfactant, we mixed the two 'species' of droplets together in a dish filled with surfactant solution and began to see some remarkable behavior emerge:
The two drops appear to chase and once caught, form asymmetric pairs that always move together in the same direction (grey drop in front, dark drop in back). Multi-drop clusters form over time, showing combinations of translational and rotational movements depending on symmetry.
But what's causing this behavior to arise?
To figure this out we examined the chemical signals each of the droplets were producing. It turned out in the surfactant solution that the predator drops were dissolving much more quickly than the prey...
To figure this out we examined the chemical signals each of the droplets were producing. It turned out in the surfactant solution that the predator drops were dissolving much more quickly than the prey...
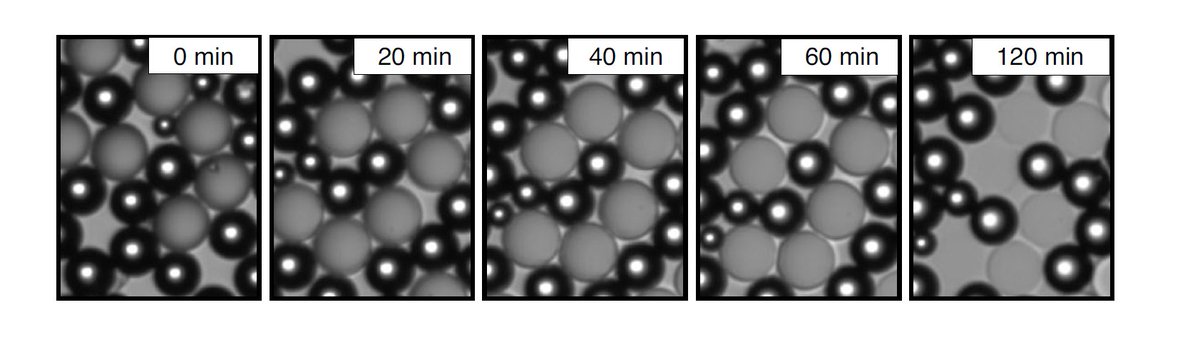
Not only that, but after watching mixed populations of droplets together over time we noticed evidence of oil transfer from the predator droplets to the prey, seen by changes their optical properties over time.
Together these observations led to our proposed mechanism: the selective dissolution of one of the oils by the surfactant results in the directional exchange of oil through the surfactant solution from predator droplets (oil source) to prey (oil sink).
To test this, we switched to a different surfactant that preferentially dissolved the other of the two oils instead. Chasing still occurred, but the roles were now reversed(!) with the former prey droplets now acting as the predator.
Because the mechanism relies on oil transfer between the drops, we also found that if we used different pairs of oils which had lower miscibility together such that oil exchange was too limited then the chasing no longer occurred.
Experimenting with other types of oils besides the initial pair we discovered we found behavior to be remarkably general and even took place between extremely similar combos of oil molecules differing by only a single methylene group! Here iodoheptane chases iodohexane:
Analyzing the interactions between individual droplet pairs we were able to develop a simple model that could reproduce and predict experimentally observed movements of multi-droplet arrangements.
In the future, we hope to be able to extend this life like behavior to other synthetic systems using similar design rules for chemical signaling (pairs of sources and sinks) and build up to larger collections of active objects that might be able to carry out collective functions.
Thanks for reading if you made it this far, I hope you enjoyed and learned something!
Special shoutout to my co-lead author Pepijn Moerman who without this work would not have been possible, the great @LaurenZarzar and the rest of the team!
Special shoutout to my co-lead author Pepijn Moerman who without this work would not have been possible, the great @LaurenZarzar and the rest of the team!

 Read on Twitter
Read on Twitter