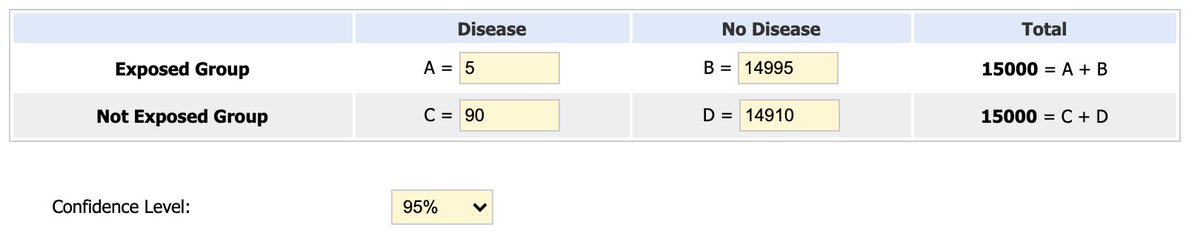
The #Modena trial. Sample size = 30,000 volunteers. 2 shots, 28 days apart. No deep freezer needed. Effectiveness claim: 94.5%. Let's look at how they got that. Reportedly, there were 95 #COVID cases: 5 in the vaccine group & 90 in the placebo group....
https://www.cdc.gov/csels/dsepd/ss1978/lesson3/section6.html
https://www.cdc.gov/csels/dsepd/ss1978/lesson3/section6.html
Vaccine effectiveness (VE) is a measure of proportionate reduction in disease among the vaccinated group. Assuming there were 15,000 people in each group, the risk of COVID infection among unvaccinated group was 0.6%. The risk among vaccinated group was 0.03%. #CovidVaccineRace
VE with this #vaccine = Risk among unvaccinated group − risk among vaccinated group, divided by risk among unvaccinated group. In this case, (90-5)/90. 85/90 = 0.94 or 94% effective. But FULL safety data is pending. What does that mean? Well.... #CovidVaccineRace #VaccinesWork
...If Modena recruited people of the right ages, races & underlying health conditions to reflect the general populations of the countries we want to vaccinate, we know ~how effective the vaccine is. To know how SAFE it is, we need to know about negative effects or bad events...
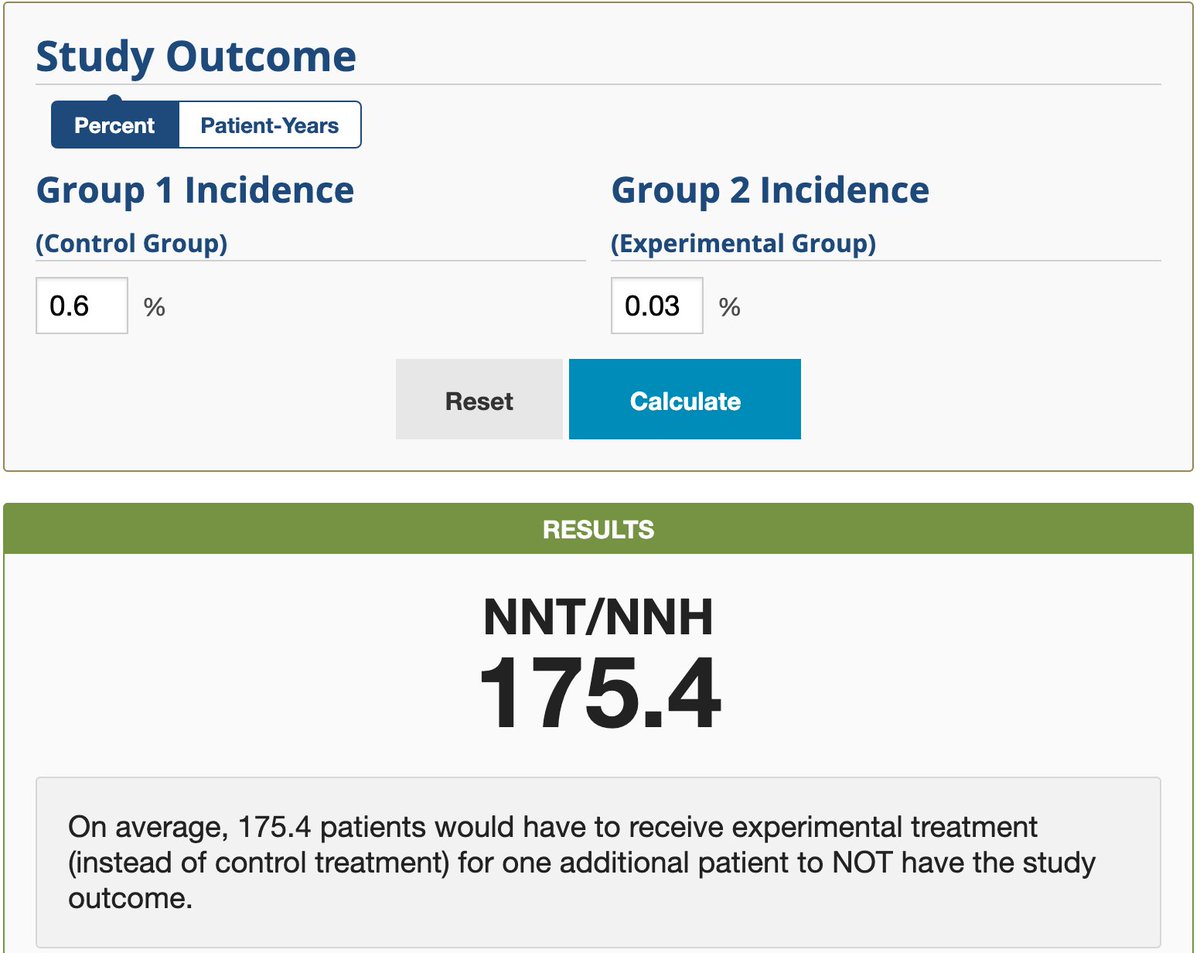
...That's the data Modena needs to deliver before we can start vaccinating people. Meanwhile, we know how many US residents we would need to vaccinate, in theory, to prevent one COVID infection: 175. This is a 2-shot series. Assuming that we won't vaccinate #COVID survivors...
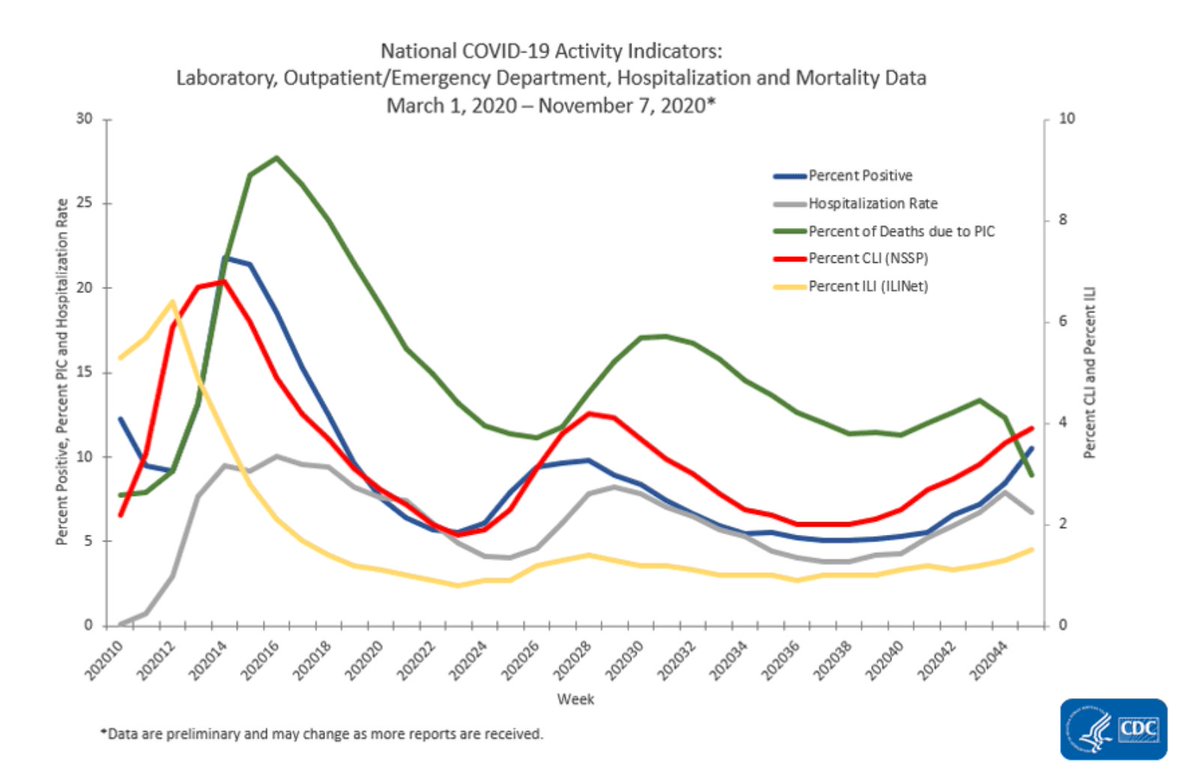
..Per the CDC, since 1/21/20 the US has had 10,846,373 cases & 244,810 deaths. 10.6 M #COVID survivors that we know of. Estimated 2020 US population: ~328M. If we want to vaccinate 317 M people, we will need 645 M vaccine doses. How long will this take to make & distribute?  ...
...
 ...
...
... Is not known. What is known? If Modena can make good on its promise of delivering 60M doses by the end of the year & we can distribute them, we still have to wait 28 days between giving both injections. And building immunity takes time. If we start vaccinating, say, Dec 1...
...by 1/31/21, we could vaccinate as many as 30 M people with the total series. If we choose to vaccinate allied health professionals first - 1.2 M practicing doctors & missions # of EMTs, Nurses, Techs - it is physically possibly cover most medical professions by Jan 31. IF...
...We dedicate enormous resources to this: money for vaccines, tests, injection kits, salaries; time to make, distribute & administer; infrastructure to inject millions of arms while socially distancing in the middle of the North American winter. Once if we give out 60 M doses...
...that might prevent as many as 171,428 infections. Which is as many as the US may have today. Preventing a day's worth of infections (especially on a bad day!) is really good. But no vaccine can or will prevent all infections. 0.06% of vaccinated people will still get COVID...
It is possible that we won't be able to safely vaccinate everyone-that data is pending. GOOD NEWS! Social distancing, mask-wearing & hand-washing can help make up the difference while we wait for ~317 M vaccine-eligible recipients to get vaccinated x2. So let's #maskup & hang on!
PS: If 60M doses are delivered nation-wide on Dec 1st, for logistical reasons, it is not likely that we can deliver them to 30M people. If we can deliver dose 1&2 to 3 M by 1/31 & dose 1 to 60 M more by, say, 2/1/21, the time-to-full distribution & immunization changes, BUT...
..Covering 94% of health professionals & high-risk folks (millions) by 1/31/21 is a worthwhile maneuver. The medical professionals will keep working & high risk folks won't end up hospitalized as often. The health system will work better, help more people & hurt fewer staff. PLUS
When vaccinated people get sick, they get much less sick. All of 11 of the severe COVID cases happened in the placebo group. SUPER good news, friends. https://www.reuters.com/article/us-health-coronavirus-vaccines-moderna/moderna-says-its-vaccine-is-94-5-effective-in-preventing-covid-19-idUSKBN27W1E6

 Read on Twitter
Read on Twitter