this study looks important:
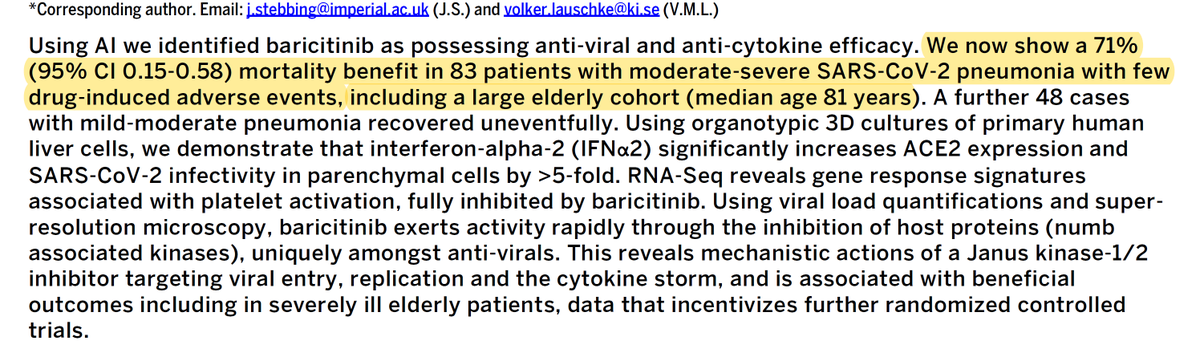
it was controlled, prospective study. it's showing a stunning 71% mortality benefit in moderate to severe covid-19 cases even in the very old.
remdesivir shows no morality benefit.
baricitinib is a widely available RA drug available in pill form.
it was controlled, prospective study. it's showing a stunning 71% mortality benefit in moderate to severe covid-19 cases even in the very old.
remdesivir shows no morality benefit.
baricitinib is a widely available RA drug available in pill form.
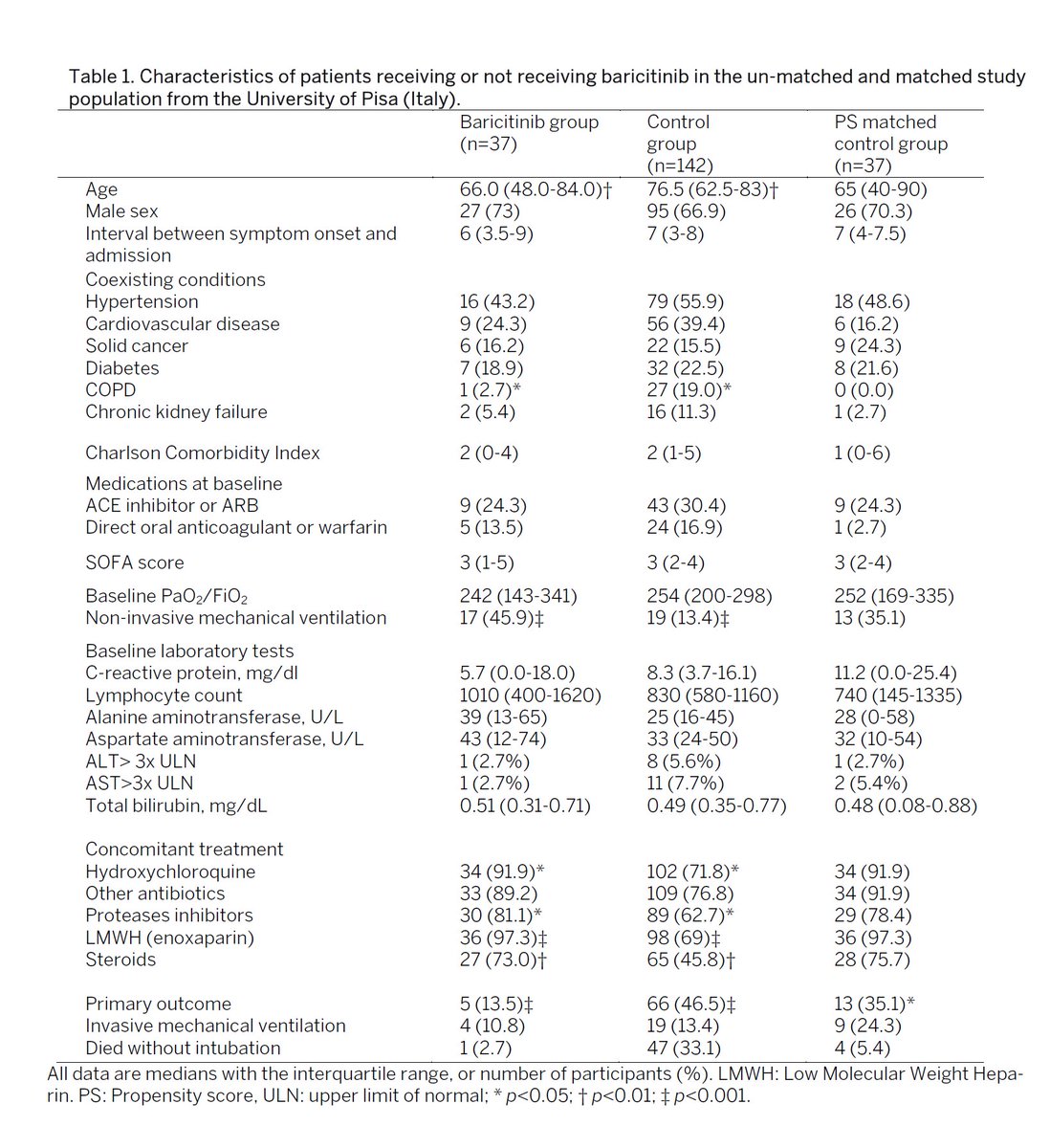
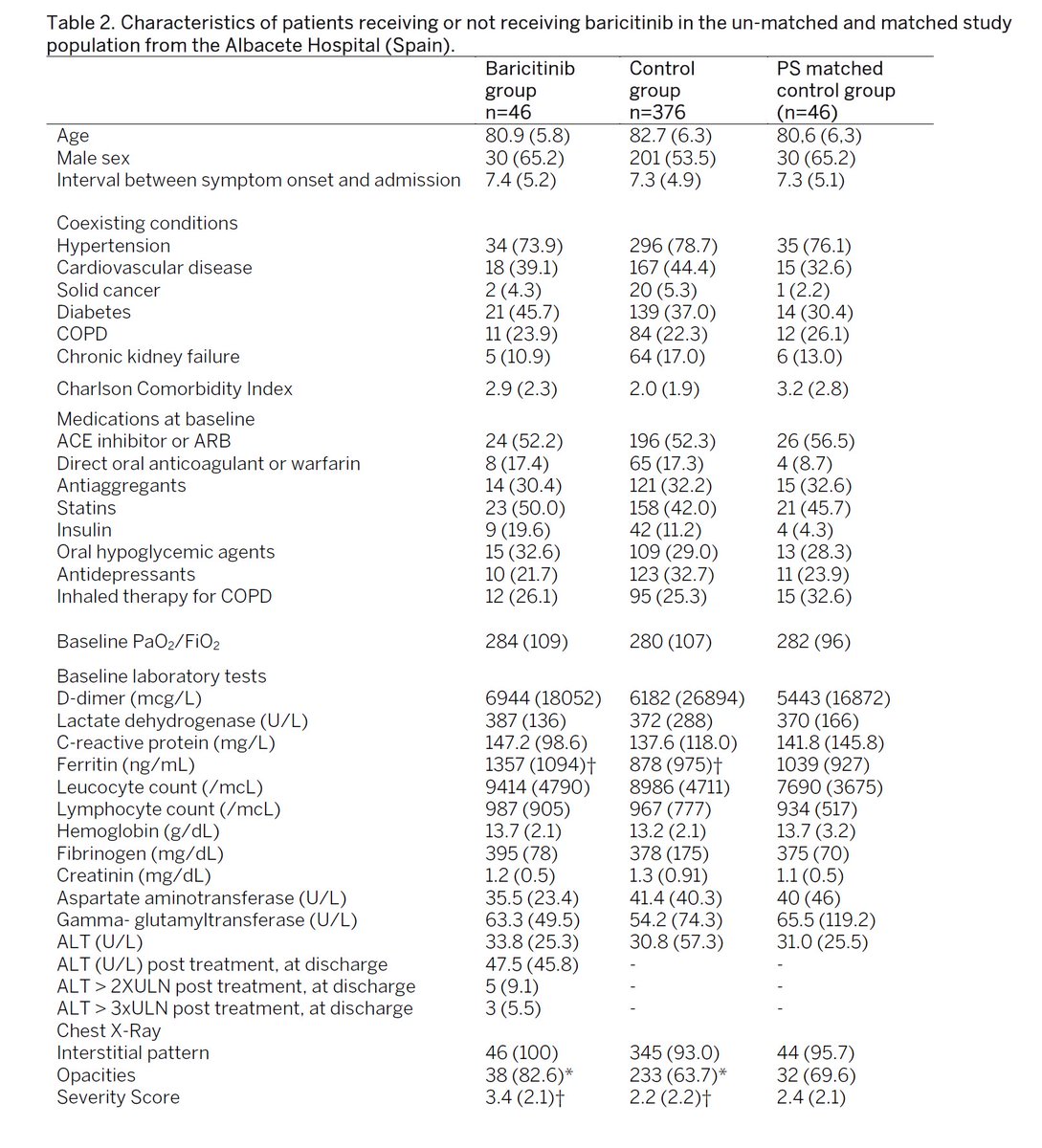
601 patients in the trial at university of pisa and albacete hospital.
83 treated with baricitinib. 83 controls established at same time using propensity score matching.
patients also received standard of care medication.
83 treated with baricitinib. 83 controls established at same time using propensity score matching.
patients also received standard of care medication.
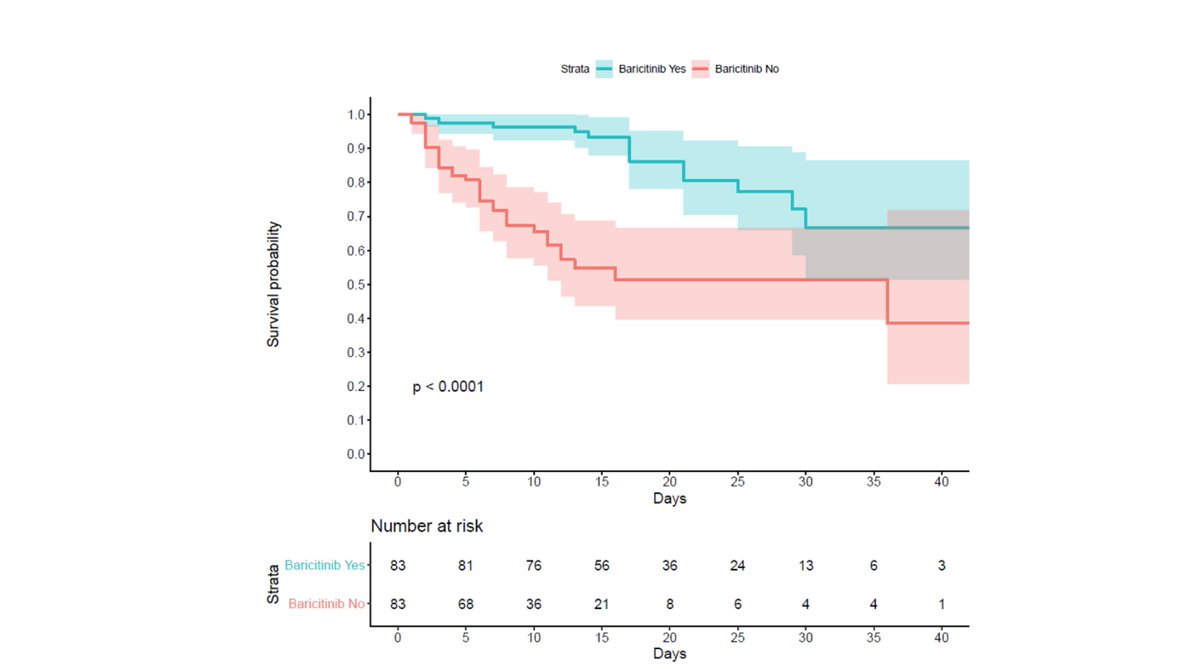
this led to dramatic differences in outcomes.
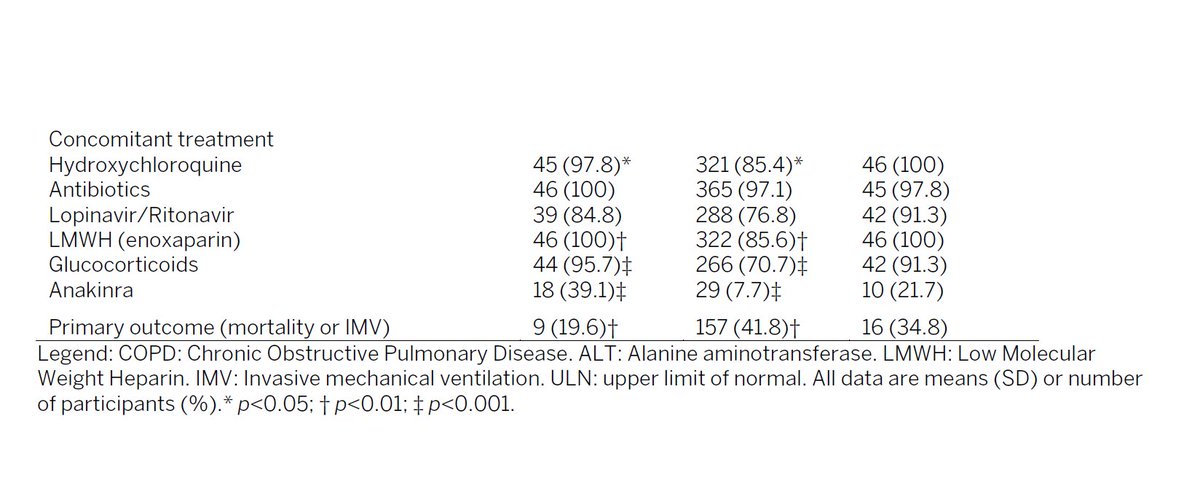
composite endpoint of death or mechanical ventilation was 29 in control group (34.9%) vs 14 in active arm (16.9%). 52% reduction. p<0.001.
effects were rapid and sustained.
curve separation was almost instant.
composite endpoint of death or mechanical ventilation was 29 in control group (34.9%) vs 14 in active arm (16.9%). 52% reduction. p<0.001.
effects were rapid and sustained.
curve separation was almost instant.
what's really striking is how efficacious this was even in the very old and sick.
avg age in the albacete group was 80.9 (baricitinib) and 80.6 (control)
that group has had very little in the way of successful treatment options
death or IMV was 9 in drug, 16 in control. p<0.01
avg age in the albacete group was 80.9 (baricitinib) and 80.6 (control)
that group has had very little in the way of successful treatment options
death or IMV was 9 in drug, 16 in control. p<0.01
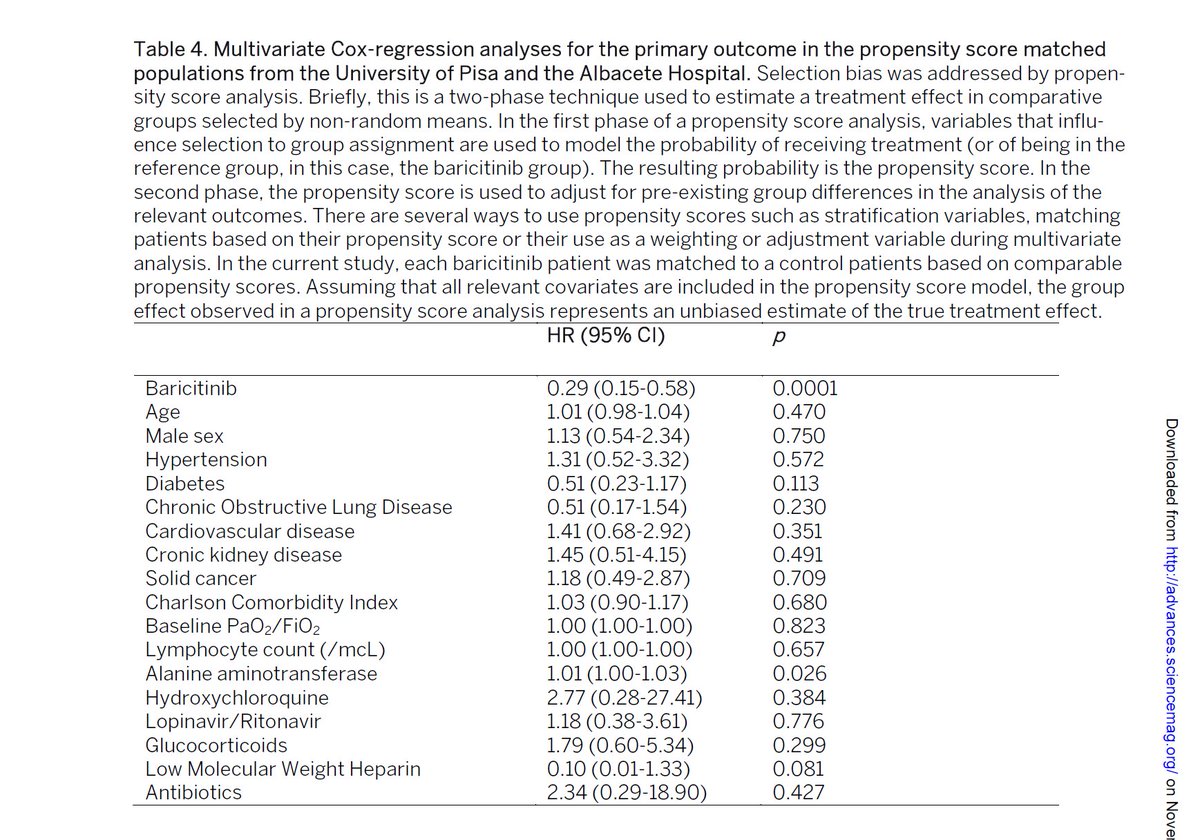
MCRA for primary outcome has drug at p =0.0001.
that is an insanely good number for such a small trial.
i read 1000's of drug trials. this is vanishingly rare.
i'd take this very seriously as an option even on just one trial.
that is an insanely good number for such a small trial.
i read 1000's of drug trials. this is vanishingly rare.
i'd take this very seriously as an option even on just one trial.
further, in prato hospital:
drug: 0% of patients in ICU 2 weeks after treatment, 78% discharged.
control: 33% of patients in ICU, 6% discharged
p = 0.01, <0.0001 respectively
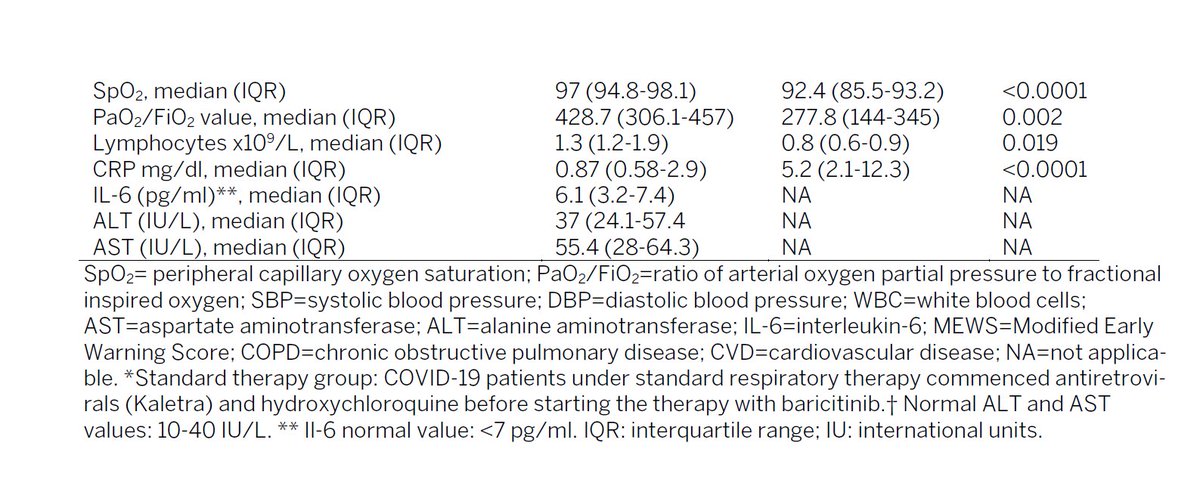
also had huge effects on key variables
97 vs 92 SpO2 is huge effect. 97 is "normal" <94 = hypoxic
drug: 0% of patients in ICU 2 weeks after treatment, 78% discharged.
control: 33% of patients in ICU, 6% discharged
p = 0.01, <0.0001 respectively
also had huge effects on key variables
97 vs 92 SpO2 is huge effect. 97 is "normal" <94 = hypoxic
all in all, this looks like a sound, prospective study design with well matched control.
it shows large and near instant separation in curves and major mortality, ventilation, and ICU benefit even in the very old who have been so vulnerable to covid.
this looks VERY promising.
it shows large and near instant separation in curves and major mortality, ventilation, and ICU benefit even in the very old who have been so vulnerable to covid.
this looks VERY promising.
and it blows remdesivir out of the water, esp for the very sick.
gilead rammed remdesivir through and the NIH panel let them change their primary endpoint right before read out to save the trial from failing.
it has no survival benefit and never did. https://www.medpagetoday.com/infectiousdisease/covid19/89167
gilead rammed remdesivir through and the NIH panel let them change their primary endpoint right before read out to save the trial from failing.
it has no survival benefit and never did. https://www.medpagetoday.com/infectiousdisease/covid19/89167
i'm sure this had nothing to do with the 8 people on the NIH panel that oversaw the trial that are paid by gilead...
71% mortality benefit and 52% drop in death/ventilation composite in an age group this vulnerable is worlds better than any other treatment i have seen here and, unlike so many, the trial looks valid.
you can read the whole paper here.
https://advances.sciencemag.org/content/advances/early/2020/11/13/sciadv.abe4724.full.pdf
you can read the whole paper here.
https://advances.sciencemag.org/content/advances/early/2020/11/13/sciadv.abe4724.full.pdf
please share this. if this confirms/plays out as described, it could be a major step forward in care and in reducing deaths among the most vulnerable.
would love to get some more takes on this data.
would love to get some more takes on this data.
the only real hole i can see here might be that the study was not randomized.
83 were selected for active arm and then 83 were matched. so it was prospective and the matching looks good unless there is some non-identified characteristic that led to initial active arm selection.
83 were selected for active arm and then 83 were matched. so it was prospective and the matching looks good unless there is some non-identified characteristic that led to initial active arm selection.
there is no obvious factor jumping out at me there, but that does not mean that such a factor could not or does not exist.

 Read on Twitter
Read on Twitter