A long thread in which I discuss our recent paper ( https://doi.org/10.1083/jcb.202002020), the @reviewcommons initiative, signing reviewer reports and a dedication to finish…
The paper was a joint effort between a great team of Marilia (a postdoc who just left my lab) and Richard (a PhD student who will shortly defend his thesis). It was very sad to see Marilia go - she is super talented (in science and art) and an incredibly hard-working team player
Marilia will be missed by everybody in the lab, but she made us a nice model to remember her by! We managed a socially-distanced gathering at work to say goodbye, but this is to be continued with a proper celebration when life returns to normal…
The paper was one of the first to be reviewed by @reviewcommons in December last year, so a few words about that. In short, a great initiative that I valued very highly. I especially loved the chance to respond first to reviewer comments and to put forward our own plan of action
As authors we are best placed to decide what experiments are worthwhile and why. It is then up to journals to decide whether they agree. In our case we had many good suggestions which helped to improve our manuscript a lot. So the review took a while, but it was time well spent
Both reviewers signed their reports, which I feel is very positive and important. We should not be afraid to be critical or to take criticisms on board. In my experience, the vast majority of reviewers are well intentioned, and transparency will improve science for us all
I signed my first review report today after much deliberation. I still have mixed feelings – like I said, I feel it is important and that is why I did it, but it still felt like a leap into the unknown. I guess change always feel a bit like that. Anyway, back to the paper…
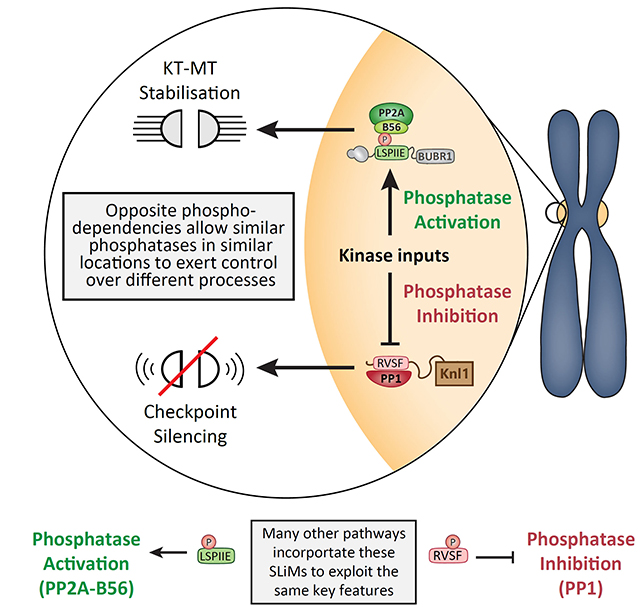
The work followed on from Richard and Marilia’s study last year where they demonstrated that PP2A and PP1 produce different effects at kinetochores by responding in opposite ways to phosphorylation inputs https://doi.org/10.1016/j.celrep.2019.07.067
That led us to focus on the inputs and, in particular, PLK1 (an activator of PP2A). What we discovered told us more about PLK1 than about the different phosphatases (although we hope this work will eventually help us to clarify PP1/PP2A differences…)
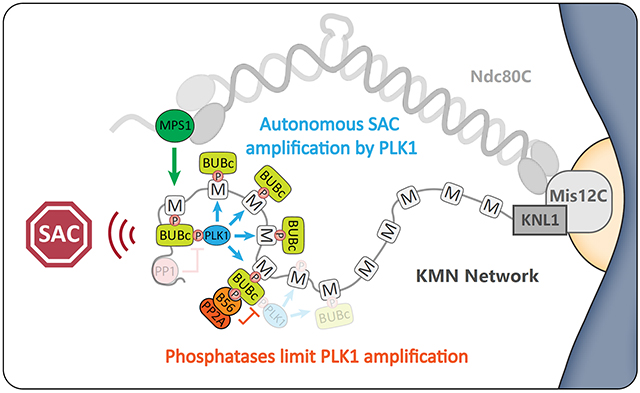
PLK1 was known to bind to phospho-residues on the BUB complex and to phosphorylate “MELT motifs” at the kinetochore (the key SAC substrates that recruit the BUBs)
We demonstrated that this BUB1-PLK1 complex acts autocatalytically to phosphorylate MELTs and therefore recruit more BUB1-PLK1. And crucially, that kinetochore phosphatases must antagonise this positive feedback loop to prevent it from locking the checkpoint signal on
Importantly, removal of PLK1 from the BUBs is the principle role of both phosphatases in the checkpoint (at least in human cells), because the PP1/PP2A inhibition phenotypes we observe can be fully rescued by inhibiting PLK1 or removing PLK1 from the BUB complex
This was a big surprise to us, because it suggests that the phosphatases are not essential to dephosphorylate the MELT motifs, as previously assumed by us and others. We speculate that another as-yet-unidentified phosphatase performs this role.
One possible explanation, which may have widespread implications, is that basal phosphatase activity exists to switch substrates off synchronously whenever kinase activity drops below a threshold. The regulated phosphatases in this case probably control the kinase threshold
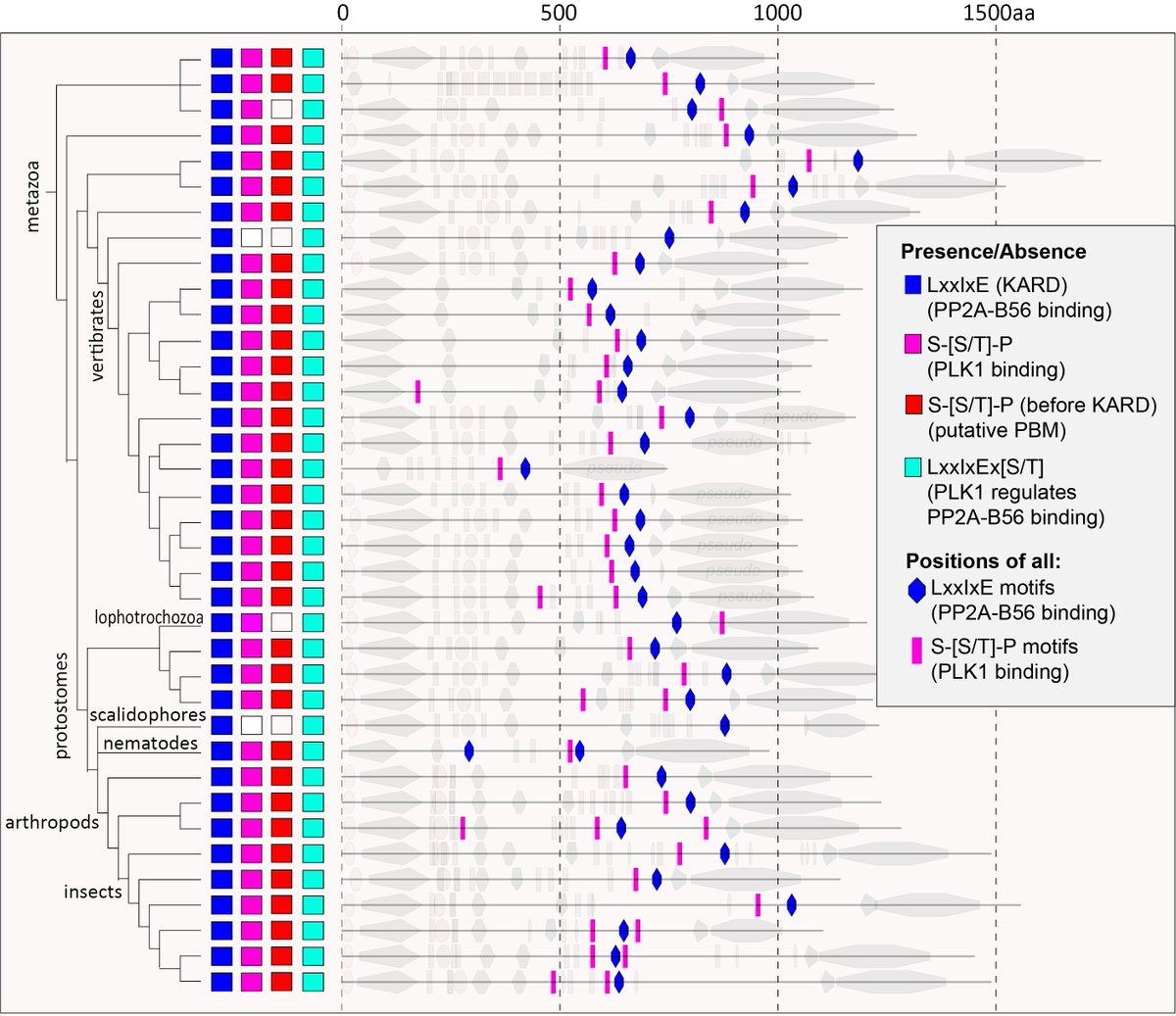
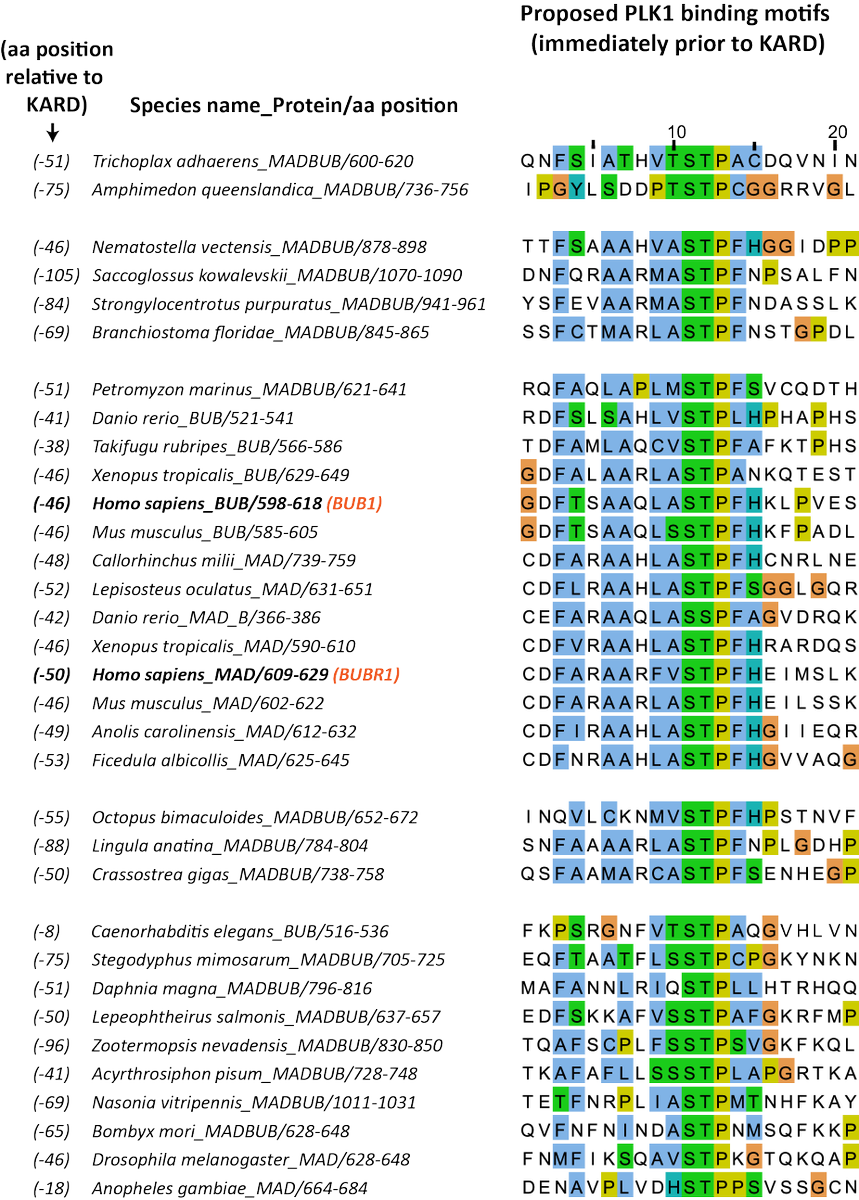
Finally, we used the excellent evolutionary analysis provided by @eelcotromer and the Snel and Kops labs to examine conservation of the PLK1-binding domain on BUB1, BUBR1 and the ancestral MADBUB homologue https://doi.org/10.1098/rsob.160315
We found that the PLK1-binding motif is strongly conserved throughout metazoa in a region immediately prior to the PP2A-binding domain. The PLK1-binding motif itself also contains well conserved hydrophobic residues, which are needed in other complexes to dimerise PLK1.
We speculate that PLK1 dimerisation may also be required at the BUB complex, inspired partly by work in press from @andreamusacchi1 lab in which they characterise the two main kinetochore receptors for PLK1 (CenpU/BUBs)
Understanding why PLK1 and PP2A modules have co-evolved in the same region on MADBUB is an important future goal and there could be many explanations for this. In addition to the checkpoint effects we uncovered, PP2A/PLK1 also regulate kinetochore-microtubule attachments
Importantly, PLK1 also phosphorylates the PP2A-binding motif to recruit PP2A. Considering PP2A then removes PLK1, this implies negative feedback between these enzymes on the BUB complex. How this complex uses such feedback is the next big question for us
I finish by dedicating this work to my dad, John Joseph Saurin. A deep thinker and a perfectionist who sadly passed away at home on Nov 5th surrounded by loving family. RIP dad. The perfectionist is never satisfied but always proud. This one is for you.

 Read on Twitter
Read on Twitter