Do the benefits outweigh the risks in using MRI gadolinium-based contrast media (GBCM) in patients with kidney disease?
MAJOR new consensus statement from @RadiologyACR and @NKF published in @Radiology_RSNA at https://doi.org/10.1148/radiol.2020202903
A #RadInTraining #TWEETORIAL thread
MAJOR new consensus statement from @RadiologyACR and @NKF published in @Radiology_RSNA at https://doi.org/10.1148/radiol.2020202903
A #RadInTraining #TWEETORIAL thread

BACKGROUND:
GBCMs consist of gadolinium ions chelated with organic ligands to minimize toxicity from free gadolinium.
Which chemical class has a lower rate of free gadolinium dissociation?
GBCMs consist of gadolinium ions chelated with organic ligands to minimize toxicity from free gadolinium.
Which chemical class has a lower rate of free gadolinium dissociation?
Answer: Macrocyclic GBCMs are more stable (lower ratio of free gadolinium to complexed ligand). (Remember, 𝐋inear is 𝐋abile)
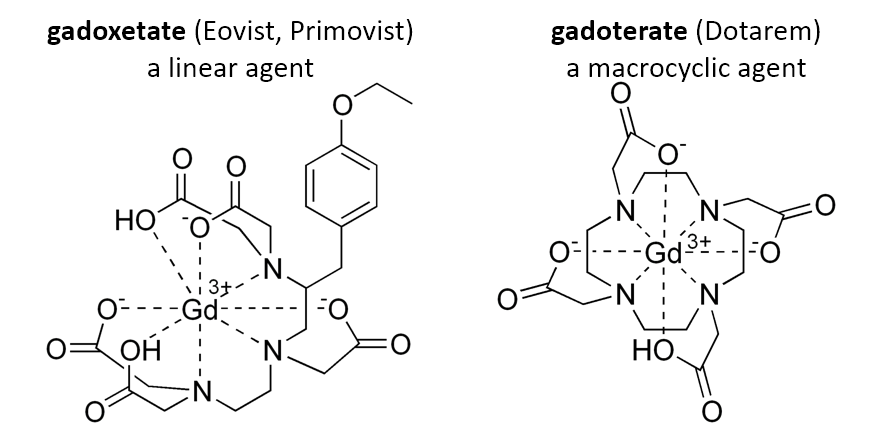
Macrocyclics form a complete ring, whereas linear agents form an incomplete ring.
Examples:
Macrocyclics form a complete ring, whereas linear agents form an incomplete ring.
Examples:
Which of the following is NOT a macrocyclic GBCM?
Nephrogenic systemic fibrosis (NSF) is a chronic fibrosing disorder, primarily affecting the skin but potentially also visceral organs (eg, lungs, heart) with fatal consequences and has been associated with GBCM exposure.
Who is most at risk of NSF?
Patients with acute kidney injury (AKI), with stage 4 or 5 chronic kidney disease (CKD), or undergoing dialysis, who have been exposed to ”group I” linear GBCMs.
 These group I linear agents are no longer popular (and have been banned in Europe).
These group I linear agents are no longer popular (and have been banned in Europe).
Patients with acute kidney injury (AKI), with stage 4 or 5 chronic kidney disease (CKD), or undergoing dialysis, who have been exposed to ”group I” linear GBCMs.
 These group I linear agents are no longer popular (and have been banned in Europe).
These group I linear agents are no longer popular (and have been banned in Europe).
That leaves the macrocyclics and the two linear agents that are not exclusively renally excreted.
“Group II” GBCMs are very low risk. These are the macrocyclics as well as gadobenate (MultiHance).
“Group III” GBCMs are also likely very low risk but confirmatory data are insufficient. This is gadoxetate (Eovist, Primovist).
“Group III” GBCMs are also likely very low risk but confirmatory data are insufficient. This is gadoxetate (Eovist, Primovist).
Let’s summarize the 3 safety categories for GBCMs per @RadiologyACR
You might be wondering, how low is “very low risk”: What is the estimated risk of NSF from group II GBCMs in patients with advanced kidney disease?
Answer: 0-0.07% is the 95% confidence interval from a meta-analysis of 4,931 group II GBCM administrations to patients with eGFR<30. There were 0 cases of NSF.
Source: Woolen et al. JAMA Int Med 2020 http://doi.org/10.1001/jamainternmed.2019.5284
Source: Woolen et al. JAMA Int Med 2020 http://doi.org/10.1001/jamainternmed.2019.5284
 KEY POINTS
KEY POINTS :
:The potential harms of delaying or withholding a group II GBCM for an indicated MRI likely outweigh the very low risk of NSF in patients with AKI or eGFR<30.
Imaging with group II GBCMs *should NOT be withheld or delayed* if harm could result from not proceeding.
Do we need to screen for impaired renal function (ie, test eGFR if clinical risk factors are present) prior to giving GBCMs?
Renal function screening is *optional* for group II GBCMs, but necessary for group III GBCMs.
Renal function screening is *optional* for group II GBCMs, but necessary for group III GBCMs.
The ACR/NKF guidelines recommend the radiologist contact the referring clinician to discuss risks/benefits/alternatives prior to administering GBCM in which of the following scenarios?
The answer is the second choice. The guideline states that, depending on individual practice patterns, group II GBCM may be administered to high-risk patients without contacting the referrer, but group III GBCMs should prompt discussion in high-risk patients (ie, AKI or GFR<30).
Is written consent required before administering GBCM to high-risk patients (AKI or eGFR<30)?
Written consent is *not required* before giving group II or III GBCMs, but patients should be informed of the risks, benefits, and alternatives.
Written consent is *not required* before giving group II or III GBCMs, but patients should be informed of the risks, benefits, and alternatives.
Should dialysis be initiated or accelerated for patients with advanced renal disease receiving GBCM?
GBCM is optimally given right before a regularly scheduled hemodialysis session but it is *not necessary* to start or alter dialysis based on group II or III GBCM administration.
GBCM is optimally given right before a regularly scheduled hemodialysis session but it is *not necessary* to start or alter dialysis based on group II or III GBCM administration.
If repeat dosing of GBCM is needed, should subsequent doses be delayed?
Waiting 24 hours permits clearance, but if there is an urgent indication, the exam *should not be delayed* for fear of NSF.
Waiting 24 hours permits clearance, but if there is an urgent indication, the exam *should not be delayed* for fear of NSF.
That’s the end of the #TWEETORIAL! These statements are going to be practice-changing. Retweet and read the full document at https://doi.org/10.1148/radiol.2020202903
#radres #MRI #radsafety #nephrology
#radres #MRI #radsafety #nephrology

 Read on Twitter
Read on Twitter