5/ I got a number of questions yesterday related to COVID-19 vaccines, vaccines in general, and the Pfizer clinical trial interim results, so I will answer several of the big ones here:
6/ Can vaccines really be 90% effective?
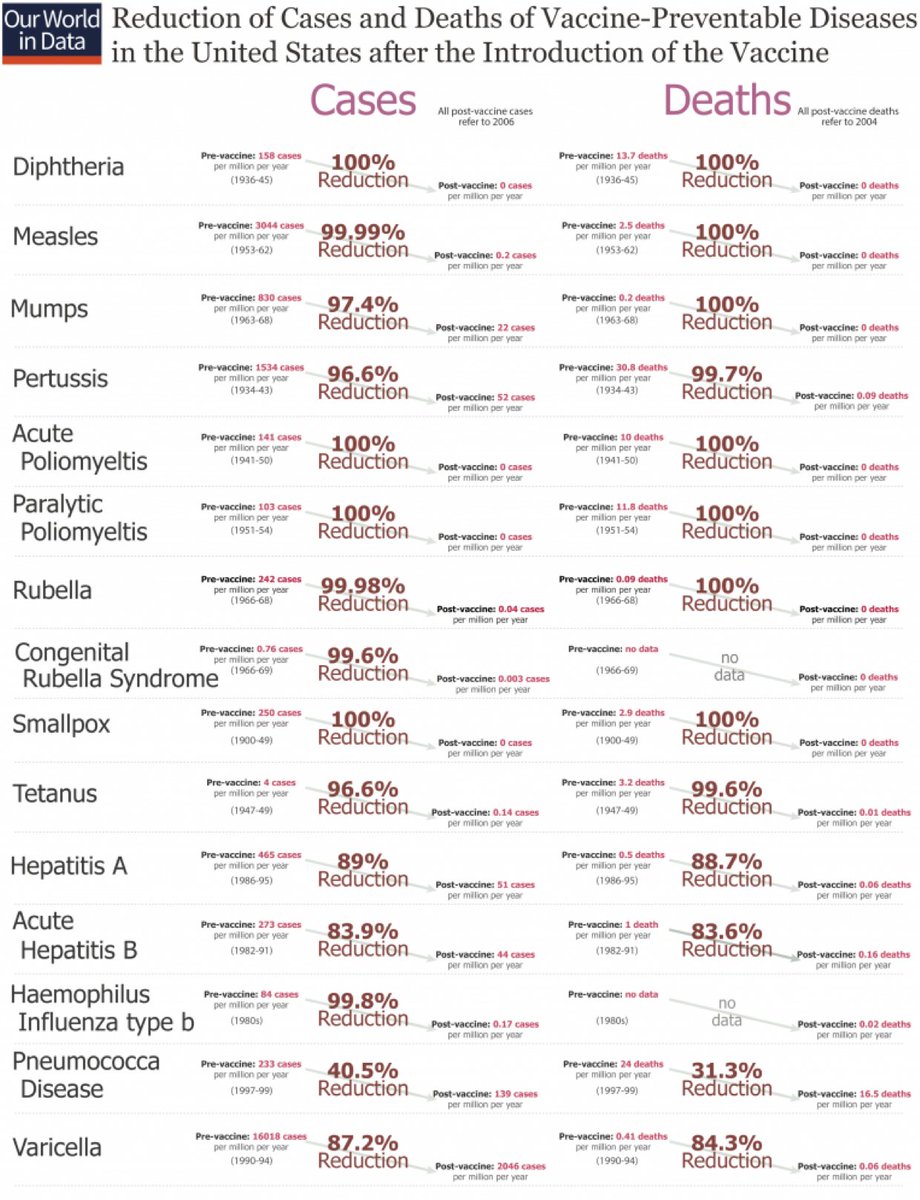
Yes, definitely! That’s one big reason vaccines are so awesome! Many of them are close to 100% effective. Smallpox, polio, measles, Diphtheria, and more!
Yes, definitely! That’s one big reason vaccines are so awesome! Many of them are close to 100% effective. Smallpox, polio, measles, Diphtheria, and more!
7/ Is the Pfizer vaccine safe?
There is an independent safety board tracking the clinical trial. The information is not all publicly available, but the vaccine has been into over 20,000 people, with two doses (so, > 40,000 immunizations), and the statement was:
There is an independent safety board tracking the clinical trial. The information is not all publicly available, but the vaccine has been into over 20,000 people, with two doses (so, > 40,000 immunizations), and the statement was:
8/ “The DMC has not reported any serious safety concerns and recommends that the study continue to collect additional safety and efficacy data as planned.”
That is consistent with the safety of the Phase 1/2 vaccine trials, which have been published and were safe.
That is consistent with the safety of the Phase 1/2 vaccine trials, which have been published and were safe.
9/ Why were there only 94 COVID-19 cases?
The short answer is that it is because the trial has been short so far. There hasn’t been much time for people to get infected after they enrolled in the trial. Based on some quick estimates, that number of cases matches expectations....
The short answer is that it is because the trial has been short so far. There hasn’t been much time for people to get infected after they enrolled in the trial. Based on some quick estimates, that number of cases matches expectations....
10/ Ok, let’s break it down:
That would be, in guesstimated terms: 100 cases per 20,000 people per 1.5 months = 33 cases per 10k per month.
Epidemiologists put it as per 100k, so, 330 cases per 100k per month.
That would be, in guesstimated terms: 100 cases per 20,000 people per 1.5 months = 33 cases per 10k per month.
Epidemiologists put it as per 100k, so, 330 cases per 100k per month.
11/ One should also assume that people who enroll in vaccine trials tend to be safety conscious; wear masks and social distance etc. So their case rate could easily be less than the average population, even though the clinical trials try to enroll higher risk individuals.
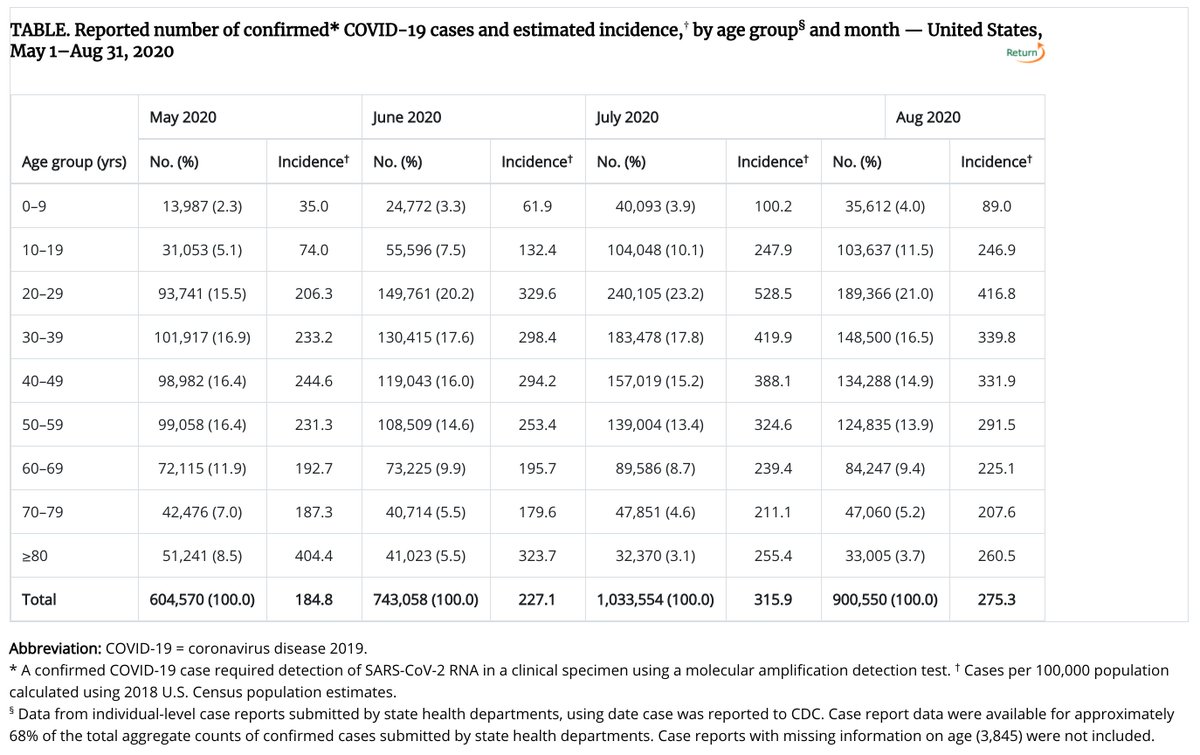
12/ The USA average for August was 27.5 cases per 10k per month (275 cases per 100k), from the CDC.
I don’t have the CDC October cases #s in front of me, but those numbers appear to be ~400 cases per 100k per month. http://USAfacts.org . https://bit.ly/3ki9ELJ
I don’t have the CDC October cases #s in front of me, but those numbers appear to be ~400 cases per 100k per month. http://USAfacts.org . https://bit.ly/3ki9ELJ
13/ So, ~330 cases per 100k per month in the placebo group seems very similar to the ~400 cases per 100k per month in the USA for October.
14/ Why is the report of symptomatic cases, not coronavirus infections?
This was decided well in advance by most of the vaccine trials. Preventing systemic disease is the #1 goal of COVID-19 vaccines. In addition, ...
This was decided well in advance by most of the vaccine trials. Preventing systemic disease is the #1 goal of COVID-19 vaccines. In addition, ...
15/ it is extraordinarily challenging to test 40,000 people for infections continuously, spread out around the country. So, instead this clinical trial (and others) have people report symptoms, and then anyone with symptoms gets a SARS2 viral test. Those are the confirmed cases.
16/ What important things have not been shown in the initial clinical trials results?
The bigs one are:
Does the vaccine prevent severe cases?
Does it work in the elderly?
How durable is the protection?
Does the vaccine prevent transmission?
But, you know, day-by-day.
The bigs one are:
Does the vaccine prevent severe cases?
Does it work in the elderly?
How durable is the protection?
Does the vaccine prevent transmission?
But, you know, day-by-day.
17/ It is a phenomenal accomplishment, for the world, to go from nothing to a vaccine with a 90% efficacy signal in ~10 months.
18/ To comment on age: The vaccine has been tested already in the older people to measure immune responses, and the results were encouraging. Antibody responses were similar in both age groups (T cell data were not reported) https://www.nejm.org/doi/10.1056/NEJMoa2027906
19/ That is also true for multiple other COVID-19 vaccines in phase 3 now, or going into phase 3 trial soon.

 Read on Twitter
Read on Twitter