THREAD 1. Today's announcement of the 90% efficacy of the Pfizer vaccine, BNT162b2, from preliminary analysis of their Phase 3 study was more great news today. So I'm writing this thread to collect in one place what I've learned so far: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-announce-vaccine-candidate-against
2. Here's the NEJM paper announcing the results of the Phase 1 trials with 195 participants: https://www.nejm.org/doi/full/10.1056/NEJMoa2027906
3. It is an mRNA (messenger RNA) vaccine, which is a recent development in vaccinology. Here's a Nature review paper that summarizes developments in the field circa ~2018: https://www.nature.com/articles/nrd.2017.243
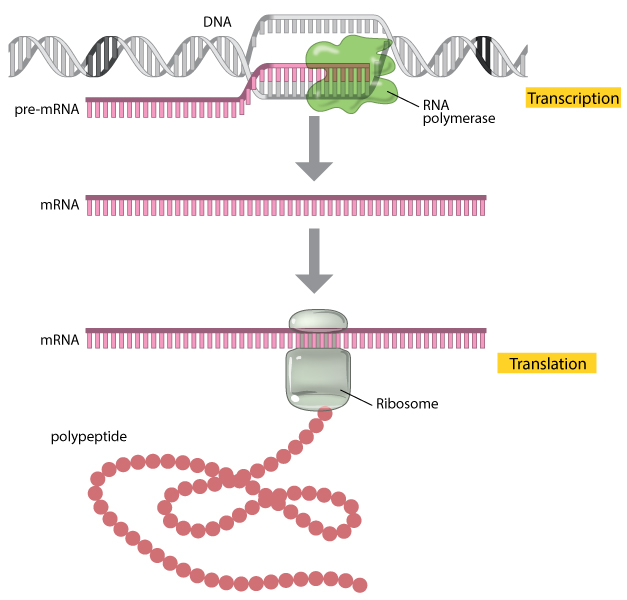
4. mRNA is the messenger molecule that is transcribed by ribosomes in a cell to generate the protein that it encodes. Here's a link to a Nature article on the overall translation process: https://www.nature.com/scitable/topicpage/translation-dna-to-mrna-to-protein-393/
5. mRNA vaccines inject mRNA analogs (modified, more stable versions of mRNA) that encodes viral proteins (in this case the SARS-CoV-2 spike protein) and gets cells in the patient to generate those proteins to stimulate an immune response. https://weekly.biotechprimer.com/abcs-of-mrna-vaccines/
6. Another mRNA vaccine under development is by Moderna, which hasn't announced results of from their ongoing Phase 3 trials yet. The NY Times is tracking different vaccine candidates here: https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html
7. The Phase 1 trial results from the Moderna vaccine were also published in NEJM earlier this year: https://www.nejm.org/doi/full/10.1056/nejmoa2022483
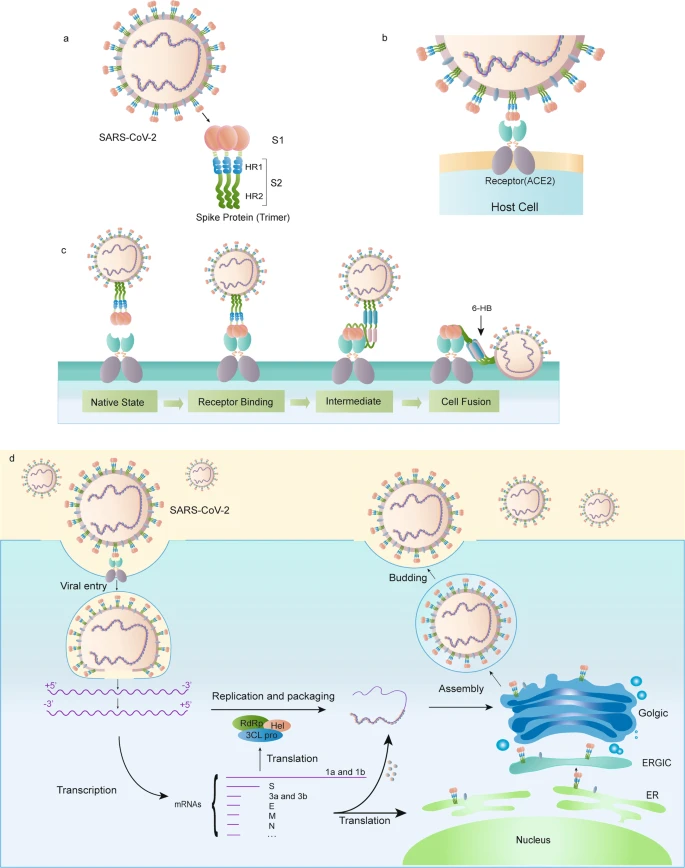
8. Both of these vaccines target the SARS-CoV-2 spike protein that is responsible for binding to the ACE2 receptor on the host. The figure below is from the Nature review paper on structure and function of the spike protein published earlier this year: https://www.nature.com/articles/s41401-020-0485-4
9. The Pfizer vaccine requires two doses, 3 weeks apart to provide immunity ~1 month after the first dose was administered. It also requires that the vaccine be stored below -70C which creates lots of logistical problems for storing and transporting the vaccine to the patients.
10. Pfizer has engineered a couple of different transport containers to avoid the need for expensive freezers at the immunization sites. Watch this excellent video to learn more about this process and what logistics companies are doing to prepare:

 Read on Twitter
Read on Twitter