Good news today with results from Pfizer/BionNtec. I thought it would be helpful to sum up what that will mean, particularly with regard to mRNA vaccines such as the one GreenLight is developing. In sum we will need more than one vaccine.
Over the next several months, I hope, we’ll see many different vaccines approved. Loosely speaking, they’ll come in three generations. The first should be licensed in the next several weeks; the second early to mid 2021, and the third batch later.
Each vaccine will have advantages and disadvantages in terms of a) Effectiveness against illness and/or infection, b) Ease of manufacturing billions of doses and availability of materials in the supply chain, c) Costs, d) Multi-dose vs single dose, e) Side-effects.
In addition, if the virus mutates, we may need to develop a new vaccine, so speed of discovery is also important.
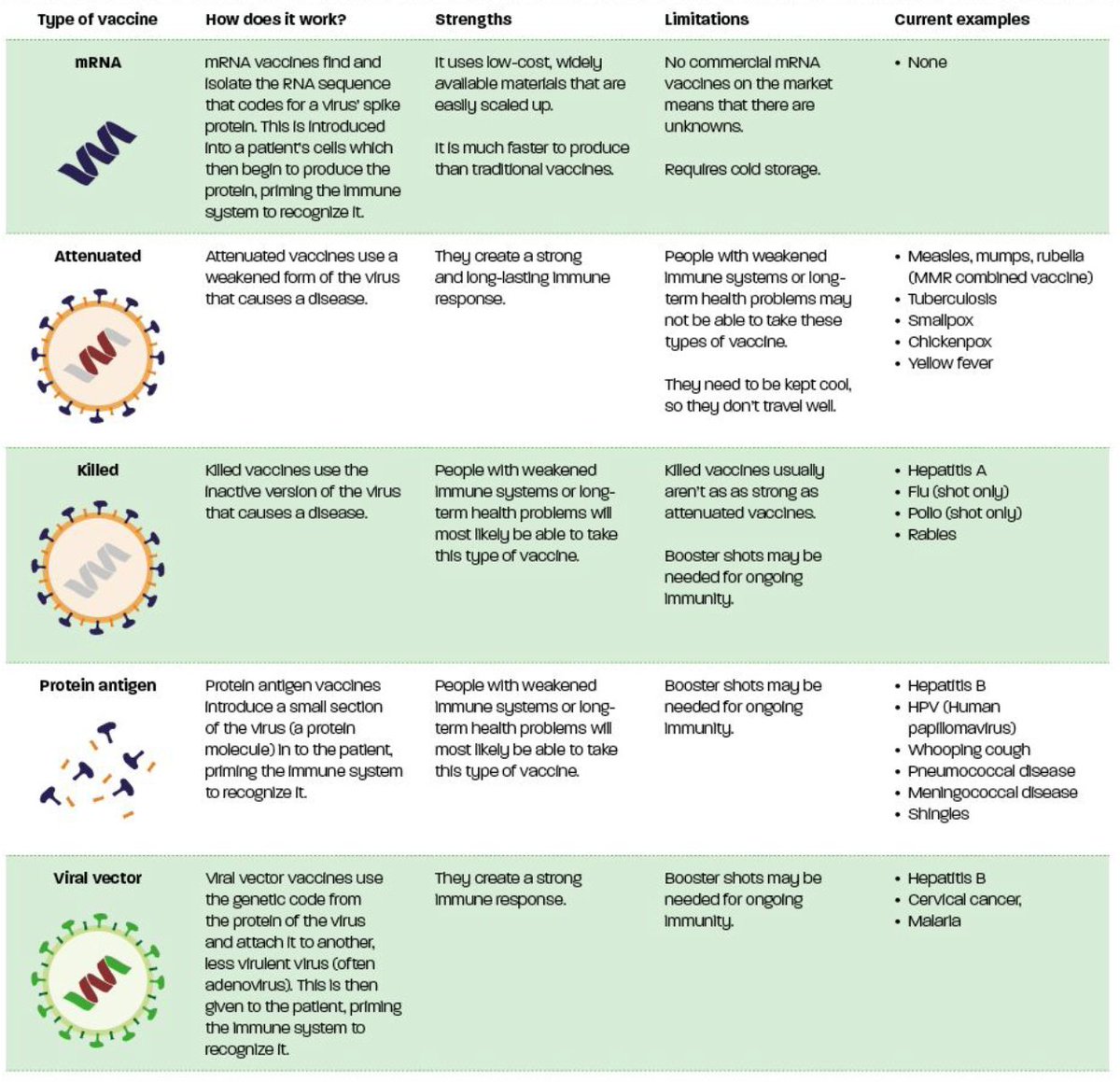
As well as arriving at different times, the vaccines will be different in how they are produced and how they work. It’s easiest to think of them in four categories: traditional vaccines, subunit vaccines, viral vector vaccines, and RNA vaccines.
All four kinds of vaccine present the immune system with an antigen that looks like the virus, so that it learns how to respond to it when it arrives for real.
Traditional vaccines use the entire virus as an antigen, either weakening it or killing it before administering it. These are reliable and well-tested, but are slow to make and need large, expensive factories.
Subunit vaccines take a part of the virus – in the case of Covid-19, the protein “spike” outside the main body of the virus – and use that as an antigen. They are much faster to produce, but the volume per dose is high, so you need to make a lot.
Viral vector vaccines also use a subunit as an antigen – again the protein spike for Covid-19. But they get the patient’s own body to manufacture it, by having a genetically modified virus instruct the body’s cells to produce the subunit. It’s much faster.
Finally, RNA vaccines use a similar process, except instead of using viruses, the genetic code to produce the subunit is packaged in tiny blobs of fat called “lipid nanoparticles”, which can get through cell membranes to the machinery within and have it produce the antigen.
RNA vaccines have the fastest discovery process. That is why the first vaccines are likely to be the RNA vaccines from Moderna and BioNTech/Pfizer (looking very likely today), assuming that they are proven to be successful in stage 3 trials.
RNA vaccines come in two main forms – self-replicating and non-self-replicating. The self-replicating forms have the body’s cells make not only the antigen, but extra copies of the RNA and its nanoparticle delivery system itself. They get the body to make its own vaccine.
Self-replicating RNA vaccines are more complex and difficult to make, but require smaller doses, so you can make doses more per batch with the same-sized bioreactor.
Roughly speaking you get ten times as much vaccine per batch (see attached link for more details): https://www.thechemicalengineer.com/features/teaming-up-for-vaccines/
The first generation of vaccines will include RNA vaccines produced by Moderna and Pfizer. They are both non-self-replicating. Imperial College’s vaccine, on track to arrive later next year, is self-replicating.
GreenLight’s own vaccine, currently in animal trials, will be non-self-replicating, and will be among the “third generation” of vaccines to arrive, if it is successful in trials.
It’s going to be important to have lots of different kinds of vaccine. Partly that’s because it’s not clear that all of them are going to work, or be safe. But partly it’s because even the ones that do work may work differently, or be best suited for different groups.
For instance, the Oxford-AstraZeneca vaccine (a viral vector vaccine) shows promising results in the elderly. It has lower levels of adverse reactions than some candidates, and creates a higher immune response: https://www.wsj.com/articles/oxford-astrazeneca-vaccine-shows-promising-immune-response-in-older-adults-11603713742?mod=djemHL_t from @jennystrasburg
Sinovac and Moderna’s vaccines, by contrast, were less effective in the elderly but had a good response in younger age groups, as @florian_krammer shows: https://twitter.com/florian_krammer/status/1310422154748612608
Kate Bingham, the head of the UK Vaccines Taskforce, has suggested that some vaccines will not prevent infection but will reduce symptoms. It may be that those are more valuable if they are used on vulnerable groups, such as the elderly: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)32175-9/fulltext
Also, different vaccines have different manufacture, delivery and storage requirements.
Traditional vaccines require highly specific manufacturing procedures, and new factories cannot be built at speed; however, they can be stored at normal refrigerator temperature for long periods.
RNA vaccines such as GreenLight’s can be produced in small, simple, prefab production units at short notice, with a chemical process that can easily be repurposed for new vaccines. However, they require storage at -80°C from manufacture almost to delivery.
There are other challenges. Almost all vaccines will need more than one dose; that is a significant hurdle. The MMR jab combines three vaccines in a single dose precisely because it is much harder to get a patient to visit the healthcare centre three times than once.
In the USA multi-dose vaccines, for HPV and Hepatitis, have sometimes had only 40% of people get fully vaccinated. We can expect people to be highly motivated next year, but this is still a challenge.
And multi-dose vaccination will be a bigger challenge in countries with less developed health systems. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4504385/
Early vaccines also seem to have mild side effects, like fevers. Even though these are temporary, they may discourage some people from coming back for a second shot. Second and third generation vaccines may fix this.
Johnson & Johnson’s viral vector vaccine is hoped to require only one dose: https://www.jnj.com/single-dose-of-johnson-johnson-covid-19-vaccine-candidate-demonstrates-robust-protection-in-pre-clinical-studies but it has been slowed down by pauses in the trial, so it's expected it will be a second-generation vaccine.
Another major concern will be producing enough of the vaccine. To achieve population immunity globally will require vaccinating a majority of the world’s citizens, probably twice each. That will mean a bare minimum of 8 billion doses.
And this needs to happen at speed. The longer it takes to vaccinate people, the longer the virus has to mutate. Existing vaccines may not work against new strains of the virus.
This is not a hypothetical concern. Already a mutant strain has arisen in mink populations in Denmark, leaving to a cull of all mink farms. And human mutation is constant.
None of the mutations, yet, cause concern, as @helenbranswell says. But the risk is there. https://www.statnews.com/2020/11/05/spread-of-mutated-coronavirus-in-danish-mink-hits-all-the-scary-buttons-but-fears-may-be-overblown/
The more people who get infected, the higher the risk of a mutation that makes vaccines less effective. https://scitechdaily.com/coronavirus-mutation-concern-verified-by-largest-covid-19-viral-sequence-analysis-in-u-s/
RNA vaccines offer the possibility of rapid, large-scale manufacture in small local facilities, which will be vital.
GreenLight aim to produce 1 billion doses over the next 18-24 months. Our process at GreenLight is relatively free of supply chain issues.
And scale-up further up to the required 8 billion doses globally thereafter.
Others think they can make 2 billion doses by the end of next year. But there may be supply chain bottlenecks. For instance, the people who supply Pfizer with their nucleotides will also supply Moderna.
RNA vaccines are going to be a vital part of the fight against Covid-19. It is vital that they are part of a wider portfolio of vaccines, because we need the vaccines we get to do many different jobs.
If you want to keep up with our work to produce a vaccine, you can sign up for email updates on our website at https://www.greenlightbiosciences.com/covid19/

 Read on Twitter
Read on Twitter