$REGN CSO George Yancopoulos presented more detail over the weekend on the company's data for the company's monoclonal antibody cocktail Covid-19 in patients who are not yet hospitalized.
A quick thread. 1/6
The full slides are here:
https://investor.regeneron.com/static-files/e128b5e8-44b0-44f4-84da-b0129a07d5f6
A quick thread. 1/6
The full slides are here:
https://investor.regeneron.com/static-files/e128b5e8-44b0-44f4-84da-b0129a07d5f6
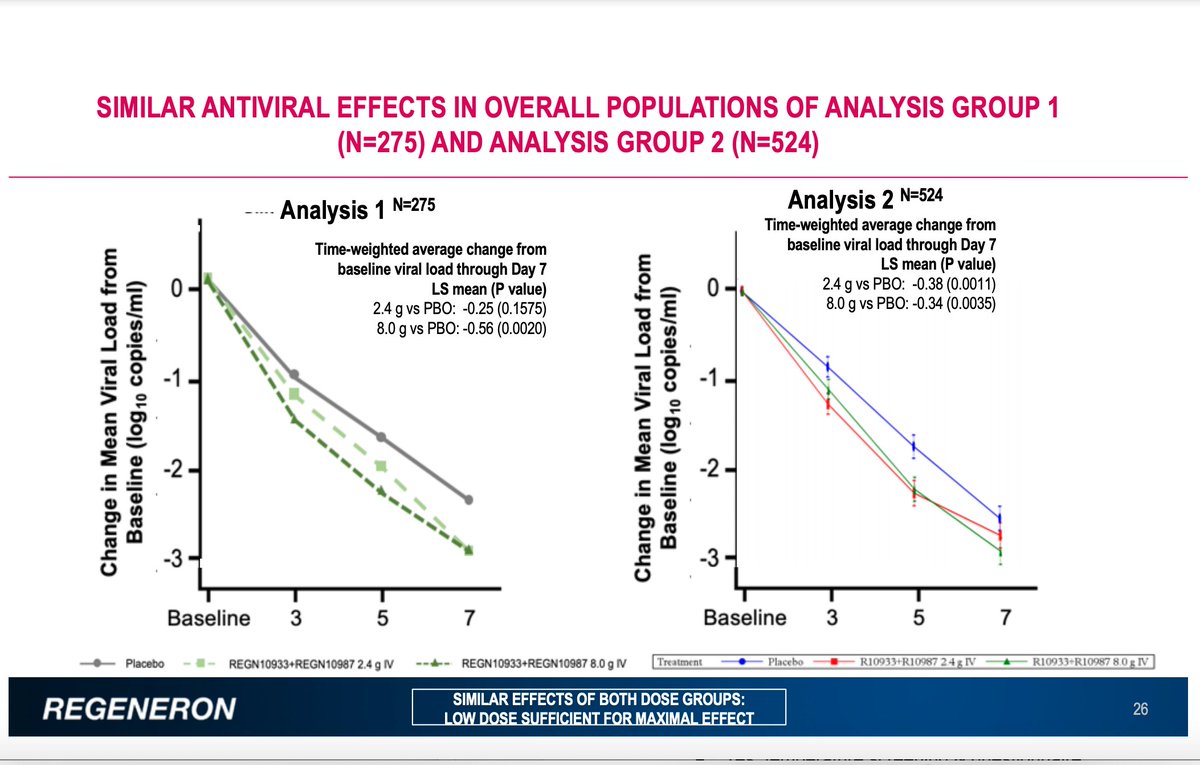
First: the antiviral effects were similar in the second group of 524 patients tested as in the first of 275. 2/6
Again, the patients who benefited the most were those who did not make enough antibodies on their own. A separate slide shows that the biggest reductions happened in patients with the highest viral loads. 3/6
The other data point there were a lot of questions about was the 60% reduction seen in doctors visits. Good news: these were mostly hospital and ER visits, not telemedicine. Bad news: Only 15 visits in the placebo group, versus 6 in each treatment group. Small numbers. 4/6
Just to be completely clear, this is different from the news from Friday, which was in patients on high-flow oxygen or ventilators. In those sicker patients, there is no benefit. 5/6
Regeneron press release: https://investor.regeneron.com/news-releases/news-release-details/regn-cov2-independent-data-monitoring-committee-recommends
Regeneron press release: https://investor.regeneron.com/news-releases/news-release-details/regn-cov2-independent-data-monitoring-committee-recommends
As I wrote last week, the challenge here is identifying who should get the treatment. The company's deal with the U.S. government provided 300,000 doses, but the U.S. is now approaching 100,000 cases a day. 6/6 https://www.statnews.com/2020/10/29/antibody-drugs-appear-effective-now-can-we-make-enough-of-them/

 Read on Twitter
Read on Twitter