Excited to share my postdoc work, just out in Science! Thanks to the labs of @SeanMegason and @HeisenbergCPLab for their support, and @BioPhysMatt , @PengXia7, Tugba, Holger for help solving the puzzle. Here is my twitter-style summary. 1/12 https://science.sciencemag.org/content/370/6512/113
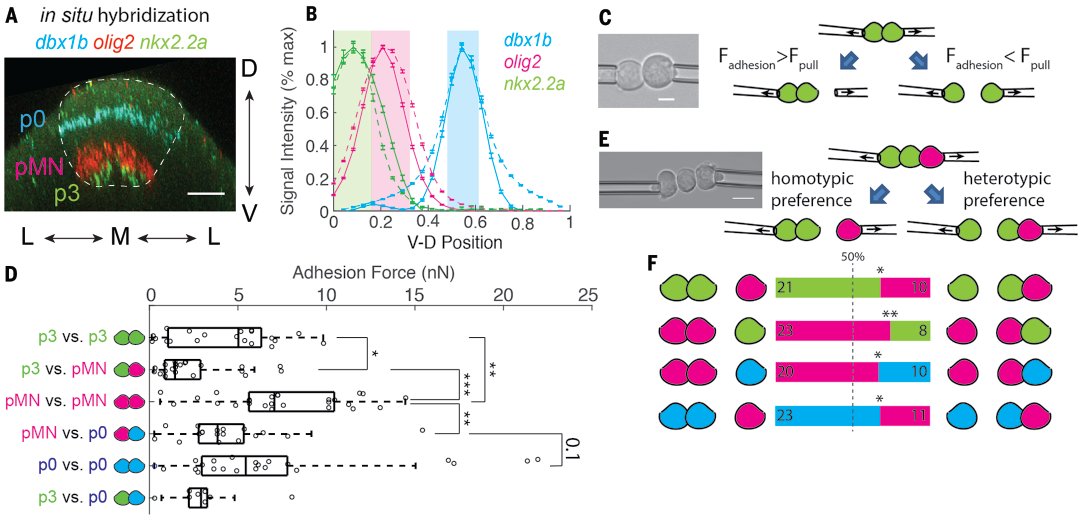
Patterns form reproducibly during embryo development, frequently in tissues rapidly changing shape. In the zebrafish spinal cord, 13 cell types form stereotypic stripe-like domains. But initial patterns are imprecise, and tissue is folding dramatically. So how is this done? 2/12
We hypothesized cells might actively seek out the right neighbor. Working with the cell mechanics expert @HeisenbergCPLab, I developed 2 assays to measure adhesion. First, the classic dual pipette assay can measure adhesion force between different types of cells. 3/12
And the triplet assay puts 2 cells of the same type and 1 of a different type in a row, providing a direct tug-of-war between the homotypic contact (between cells of the same type) and the heterotypic contact (cells of different type). 4/12
Turns out in all 3 cell types I tested, they all have stronger adhesion force with cells of the same type, and prefer to adhere to cells of the same type in the tug-of-war assay. 5/12
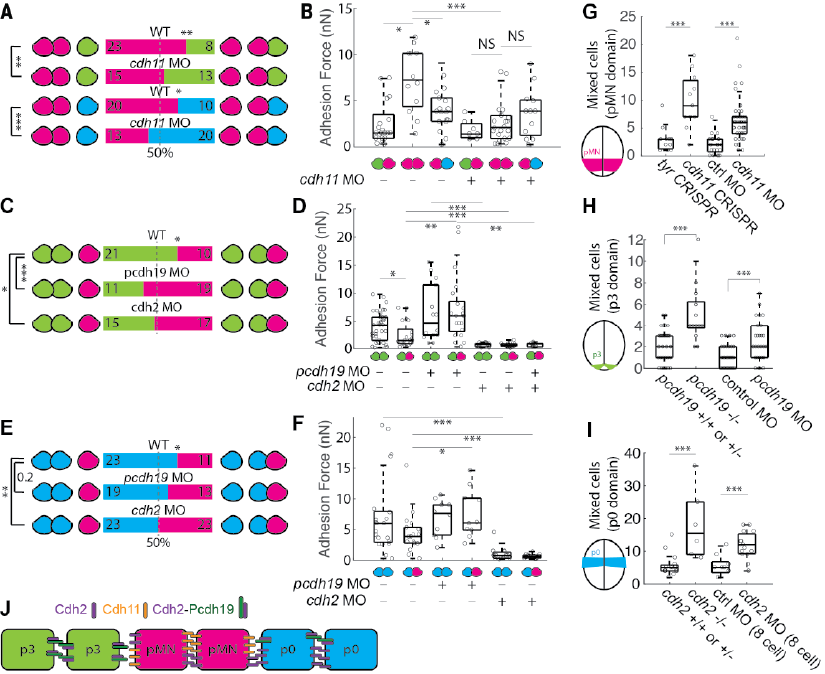
So how do the cells achieve this adhesion specificity? Combining transcriptomic analysis and CRISPR genetics, we narrowed down to 3 adhesion molecules, N-cadherin, cadherin 11, and protocadherin 19. They form a combinatorially unique “code” in each cell type. 6/12
We systemically remove these adhesion molecules to test how they affect different types of homotypic/heterotypic adhesion. It appears that each cell type is using a different mode of adhesion to help them achieve adhesion specificity. 7/12
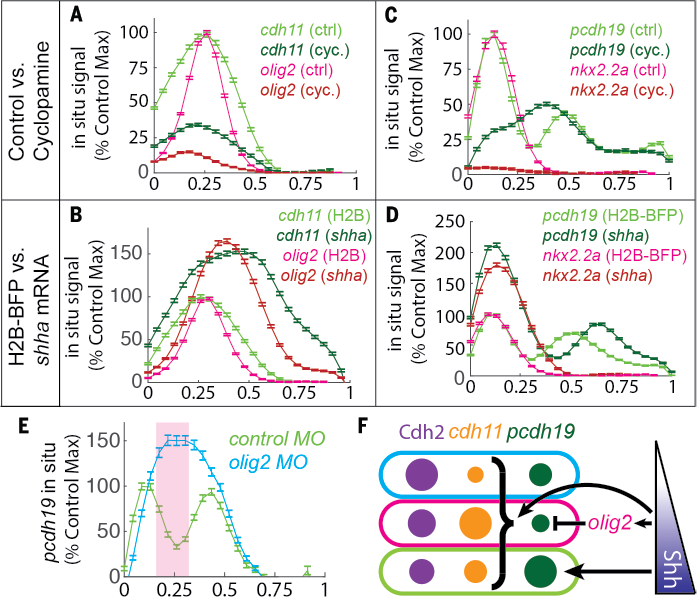
Finally, how do the cells know which adhesion molecule to make? Turns out the adhesion code is controlled by the Shh morphogen gradient, which also controls cell fate. So this puts differential adhesion under the control of a morphogen gradient! 8/12
From the perspective of robust patterning, patterns formed with only morphogen gradient could result in imprecise boundary because of signal noise. Patterns formed with only adhesion-based self-organization solve the boundary issue but lack precision in global orientation. 9/12
The collaboration between morphogen gradient and differential adhesion allows cells to sense global tissue orientation, while refining local patterns and structures. This could be useful to think about how patterns form robustly during morphogenesis in general. 10/12
Besides work in the Megason lab at HMS sysbio, I spent a total of 9 months over 4 years in @HeisenbergCPLab, which is instrumental for this work. These trips won’t be possible without the generous funding from @DamonRunyon, @Co_Biologists, @BWFUND, and @NICHD_NIH. 11/12
Finally, I am starting my lab @WashUDevBio @WUSTL
on Jan 2021. We will explore how interplay between morphogen signaling and mechanics help make pattern formation and morphogenesis robust in zebrafish embryos. We are recruiting at all levels, please msg me if interested! 12/12
on Jan 2021. We will explore how interplay between morphogen signaling and mechanics help make pattern formation and morphogenesis robust in zebrafish embryos. We are recruiting at all levels, please msg me if interested! 12/12

 Read on Twitter
Read on Twitter