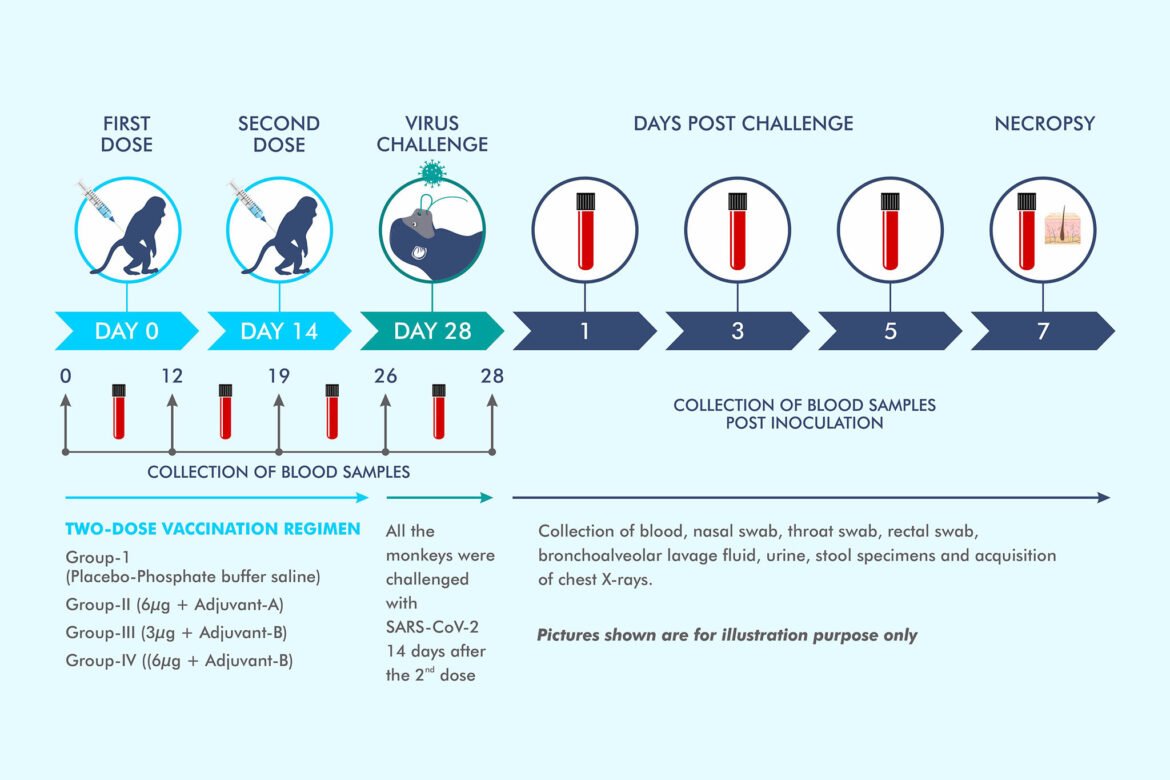
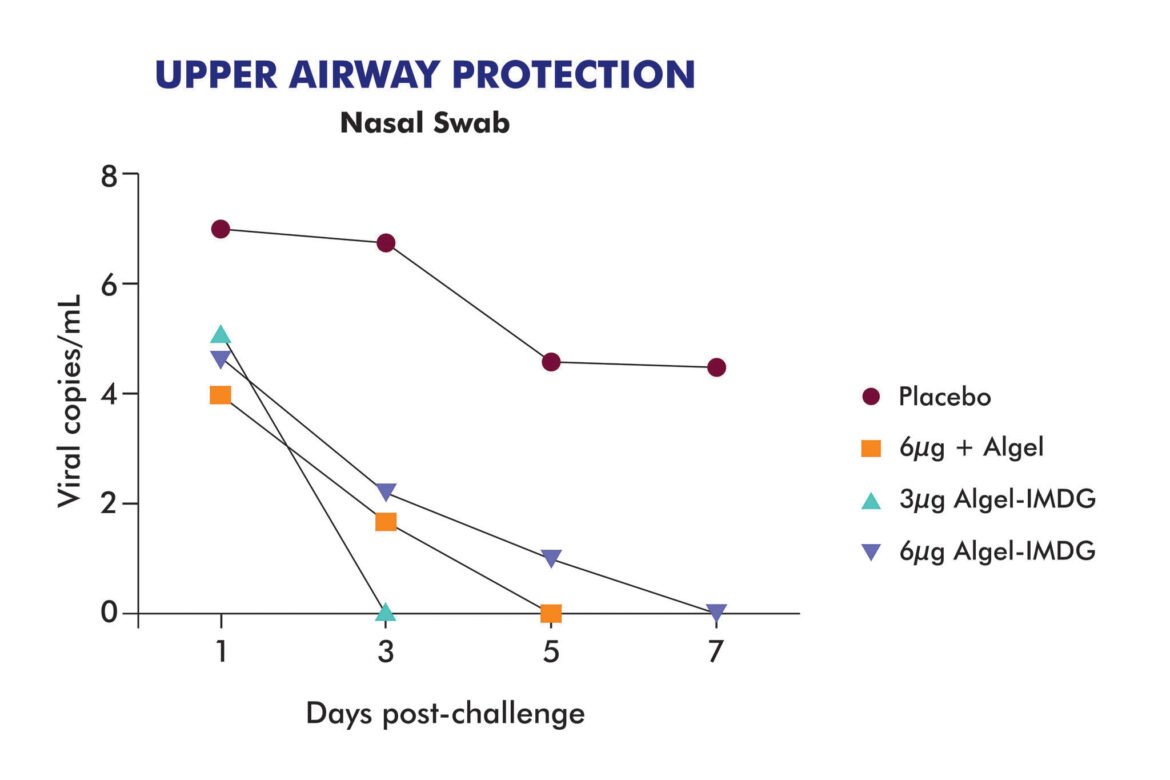
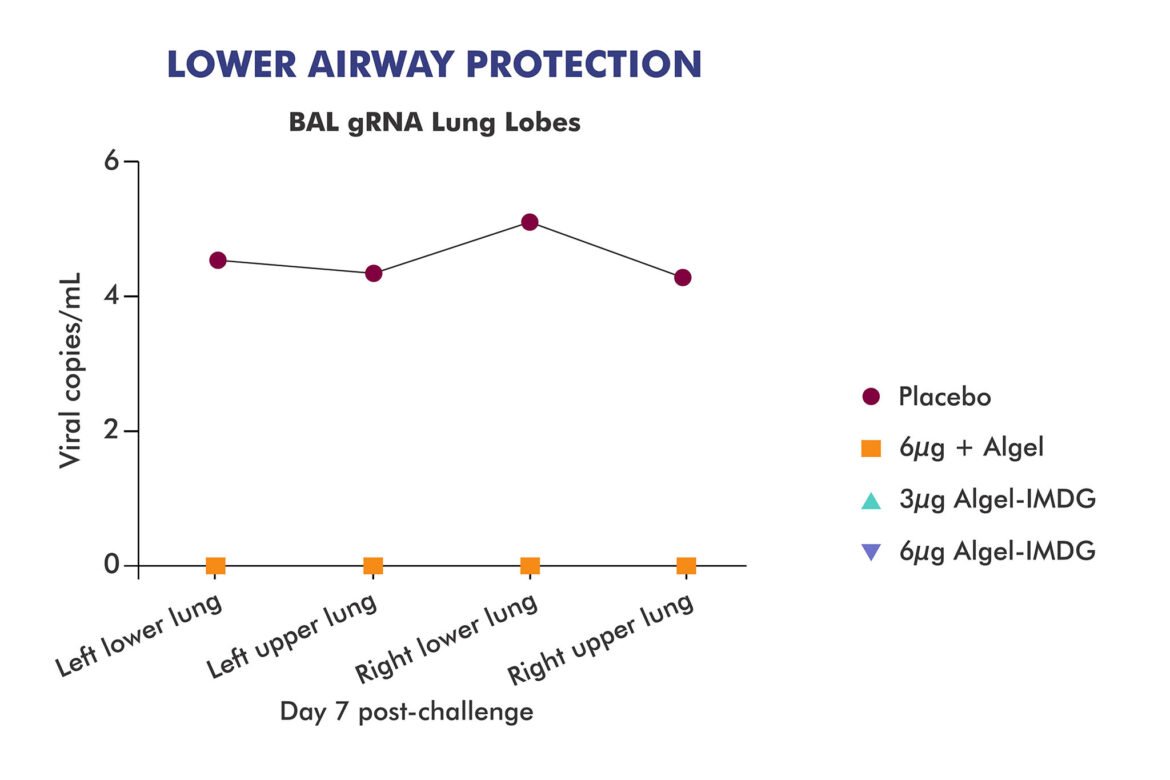
@BharatBiotech's COVAXIN (COVID19 vaccine) trial results in 20 rhesus macaques is out. https://www.bharatbiotech.com/blog/vaccines/covaxin-animal-study-results/
https://www.researchsquare.com/article/rs-65715/v1
@malini_aisola @drkamnakakkar @prat1112001 @nowme_datta https://twitter.com/BharatBiotech/status/1304413008756531201
https://www.researchsquare.com/article/rs-65715/v1
@malini_aisola @drkamnakakkar @prat1112001 @nowme_datta https://twitter.com/BharatBiotech/status/1304413008756531201
#COVAXIN @BharatBiotech A/c to news source, Phase I trial had begun by late July 2020 itself: https://www.ndtv.com/india-news/coronavirus-vaccine-covaxin-man-30-gets-first-dose-of-undertrial-covid-vaccine-in-delhis-aiims-as-human-trial-begins-2268579
Dr Guleria explaining about Phase I trial https://www.indiatoday.in/india/video/vaccine-will-be-tested-on-375-volunteers-in-phase-1-dr-randeep-guleria-on-covaxin-trials-1702521-2020-07-20
@malini_aisola
Dr Guleria explaining about Phase I trial https://www.indiatoday.in/india/video/vaccine-will-be-tested-on-375-volunteers-in-phase-1-dr-randeep-guleria-on-covaxin-trials-1702521-2020-07-20
@malini_aisola
@BharatBiotech: Phase II clinical trial of COVAXIN is ongoing.
Where are results from Phase I clinical trial, @BharatBiotech? Can we be transparent? @malini_aisola @prat1112001 @pash22 @d_s_thakur @RemaNagarajan @dawalelo @namita_kohli @VidyaKrishnan https://twitter.com/BharatBiotech/status/1304368014024876032
Where are results from Phase I clinical trial, @BharatBiotech? Can we be transparent? @malini_aisola @prat1112001 @pash22 @d_s_thakur @RemaNagarajan @dawalelo @namita_kohli @VidyaKrishnan https://twitter.com/BharatBiotech/status/1304368014024876032
" @ICMRDELHI has transferred the #Covid_19 strain isolated at
@icmr_niv to @bharatbiotech. We will be partnering with them to develop an indigenous #Covid_19 vaccine!" tweets ICMR on May 09, 2020. https://pib.gov.in/PressReleseDetailm.aspx?PRID=1622233#.XrVx6omwAv0.twitter press brief on Approval of project
https://twitter.com/ICMRDELHI/status/1259139533754400769
@icmr_niv to @bharatbiotech. We will be partnering with them to develop an indigenous #Covid_19 vaccine!" tweets ICMR on May 09, 2020. https://pib.gov.in/PressReleseDetailm.aspx?PRID=1622233#.XrVx6omwAv0.twitter press brief on Approval of project
https://twitter.com/ICMRDELHI/status/1259139533754400769
Human trials started by mid-July. Phase II trial is going on.
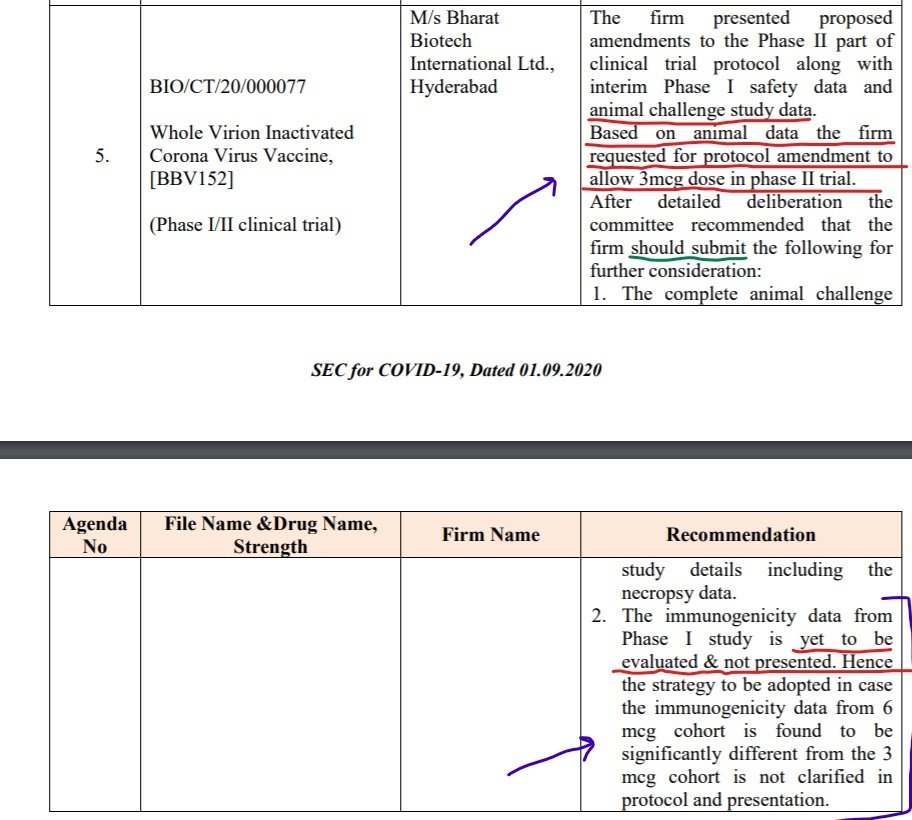
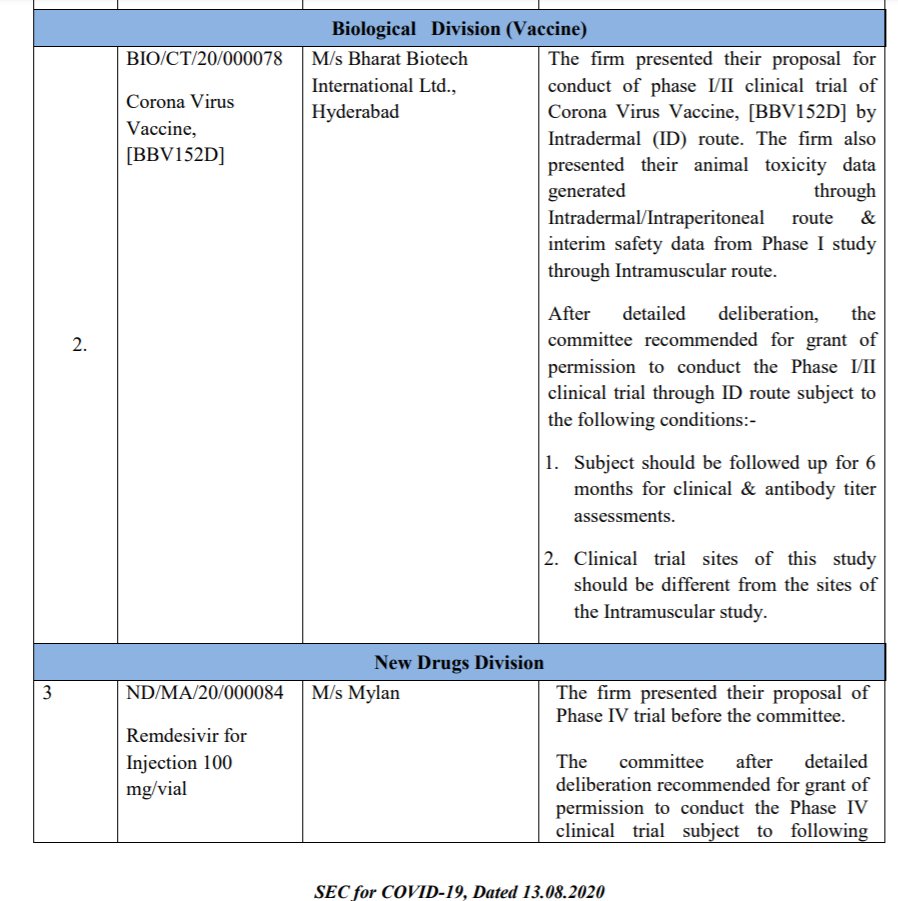
On Sept 01, 2020: @BharatBiotech presented interim Phase I safety data and animal challenge study data to SEC. Based on animal data the firm requested for protocol amendment to allow 3mcg dose in phase II trial.
On Sept 01, 2020: @BharatBiotech presented interim Phase I safety data and animal challenge study data to SEC. Based on animal data the firm requested for protocol amendment to allow 3mcg dose in phase II trial.
 Data from Sputnik-V trials in Russia have been flagged by scientists. https://www.nature.com/articles/d41586-020-02619-4
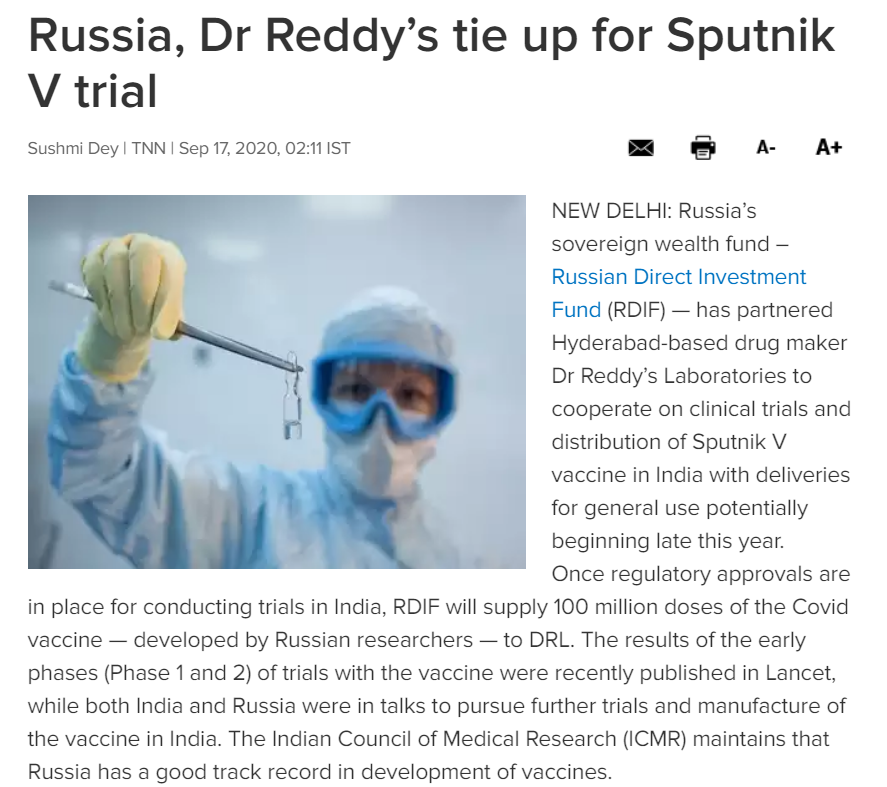
Data from Sputnik-V trials in Russia have been flagged by scientists. https://www.nature.com/articles/d41586-020-02619-4  Russian Direct Investment Fund partnered w/ Dr Reddy's Laboratories to cooperate on clinical trials & distribution of Sputnik-V (COVID19) vaccine in India. https://timesofindia.indiatimes.com/india/russia-dr-reddys-tie-up-for-sputnik-v-trial/articleshow/78157446.cms
Russian Direct Investment Fund partnered w/ Dr Reddy's Laboratories to cooperate on clinical trials & distribution of Sputnik-V (COVID19) vaccine in India. https://timesofindia.indiatimes.com/india/russia-dr-reddys-tie-up-for-sputnik-v-trial/articleshow/78157446.cms
 State govts have received GOI's directive to prepare the HS/PHC/District level micro plan for conducting vaccination against COVID19 in near future.
State govts have received GOI's directive to prepare the HS/PHC/District level micro plan for conducting vaccination against COVID19 in near future. via @malini_aisola
@pash22 @d_s_thakur @MenonBioPhysics @prat1112001 @shilpajn @Jairam_Ramesh
https://twitter.com/das_seed/status/1305813675090022401
Web portal of COVID19 vaccines related information by @ICMRDELHI: https://vaccine.icmr.org.in/covid-19-vaccine https://twitter.com/ICMRDELHI/status/1310546044166443008
Not a single paper on COVID19 vaccine trials on humans from @BharatBiotech/ @ZydusUniverse BUT @CDSCO_INDIA_INF has approved their Phase III trials. Even SEC hasn't reviewed complete Phase I (& Phase II) data yet! 

@prat1112001 @pash22 @GorwayGlobal
https://twitter.com/das_seed/status/1292909562916491265


@prat1112001 @pash22 @GorwayGlobal
https://twitter.com/das_seed/status/1292909562916491265
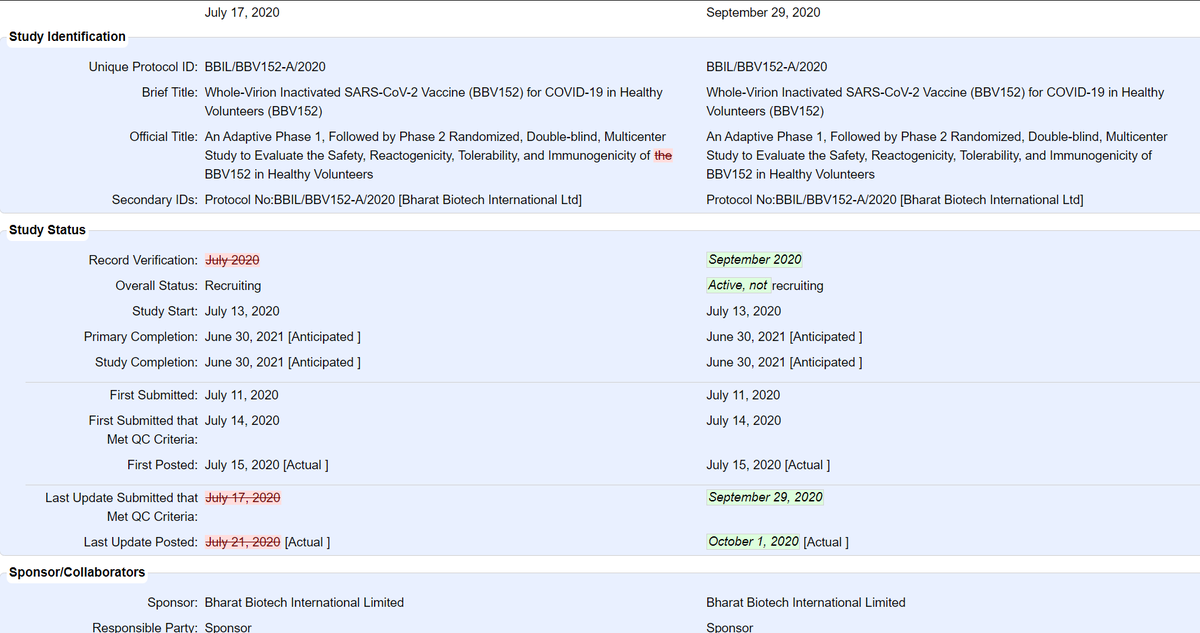
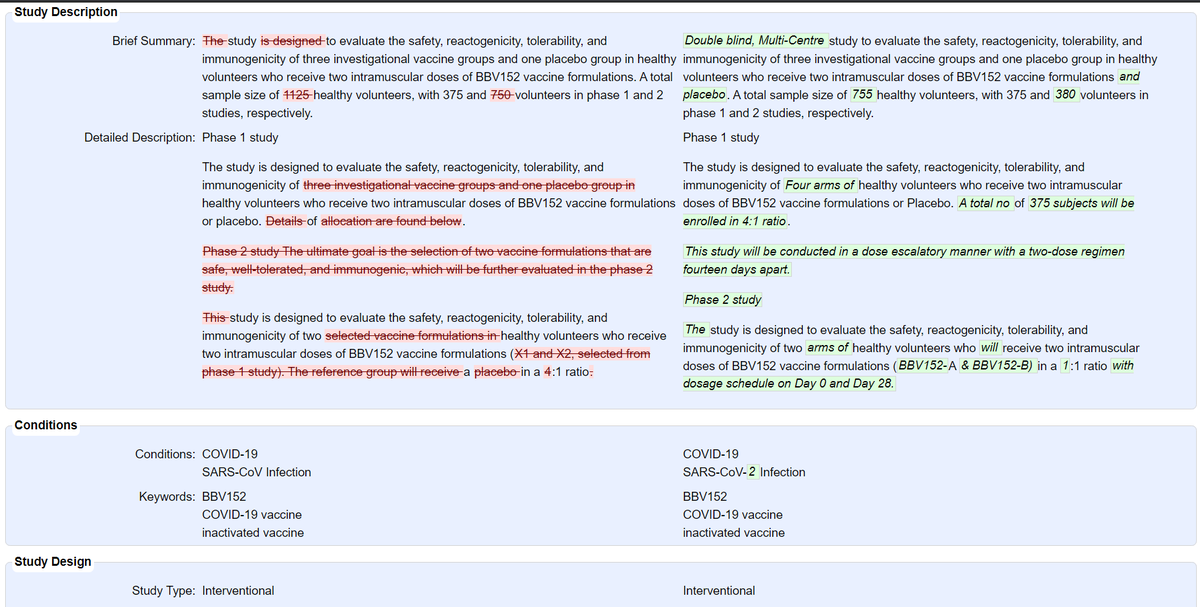
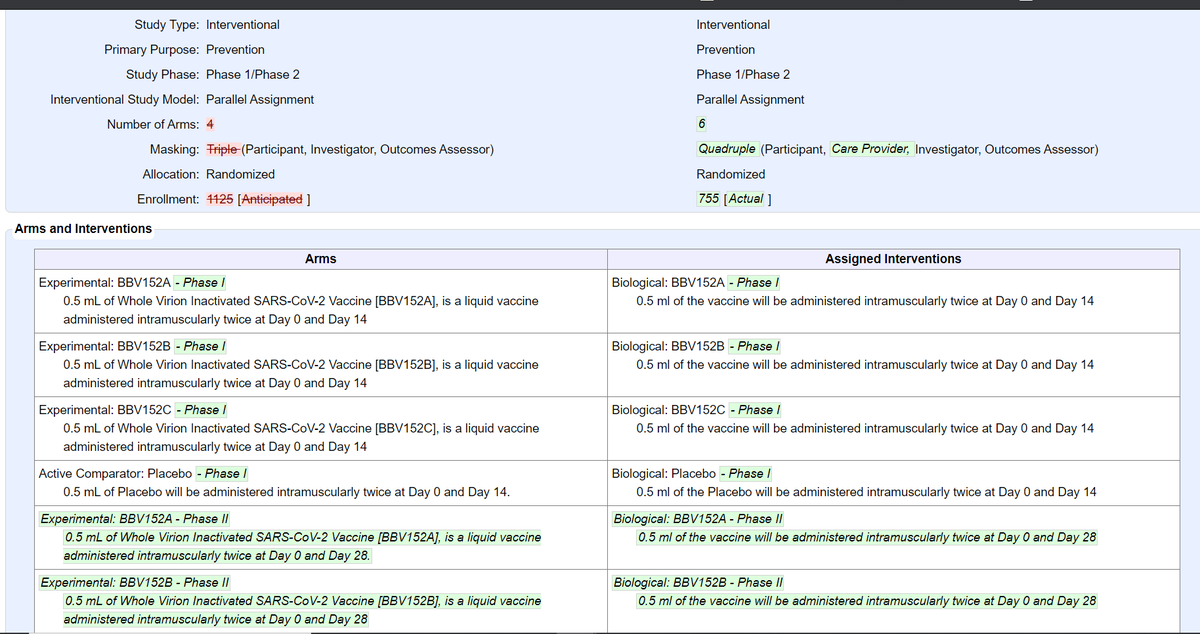
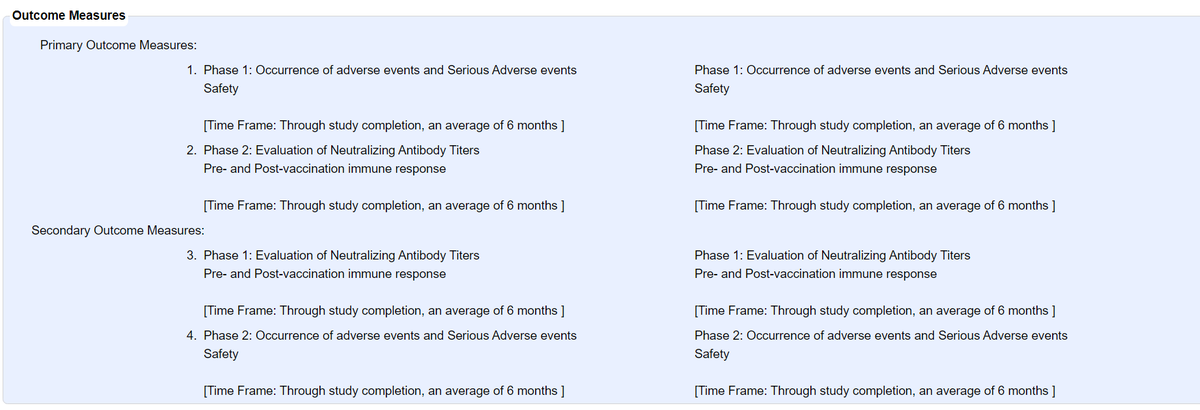
 @BharatBiotech's COVID19 vaccine: 755 participants (375 in Phase I, 380 in Phase II) in total. Phase II is double-blind, randomized (but not controlled) w/ 2 arms (1:1) for BBV152A & BBV152B - Phase II
@BharatBiotech's COVID19 vaccine: 755 participants (375 in Phase I, 380 in Phase II) in total. Phase II is double-blind, randomized (but not controlled) w/ 2 arms (1:1) for BBV152A & BBV152B - Phase IIupdate: Jul 17 vs Sep 29
 https://clinicaltrials.gov/ct2/show/NCT04471519 https://twitter.com/das_seed/status/1317111298941923329
https://clinicaltrials.gov/ct2/show/NCT04471519 https://twitter.com/das_seed/status/1317111298941923329
@SerumInstIndia has begun manufacturing Codagenix CDX-005 which is an intranasal, live-attenuated vaccine candidate for SARS-CoV-2: @drharshvardhan
@malini_aisola @RemaNagarajan @ChandnaHimani @namita_kohli @pash22 @d_s_thakur @nuts2406 @grumpeoldman https://mobile.twitter.com/PIB_India/status/1317751287350317058
@malini_aisola @RemaNagarajan @ChandnaHimani @namita_kohli @pash22 @d_s_thakur @nuts2406 @grumpeoldman https://mobile.twitter.com/PIB_India/status/1317751287350317058
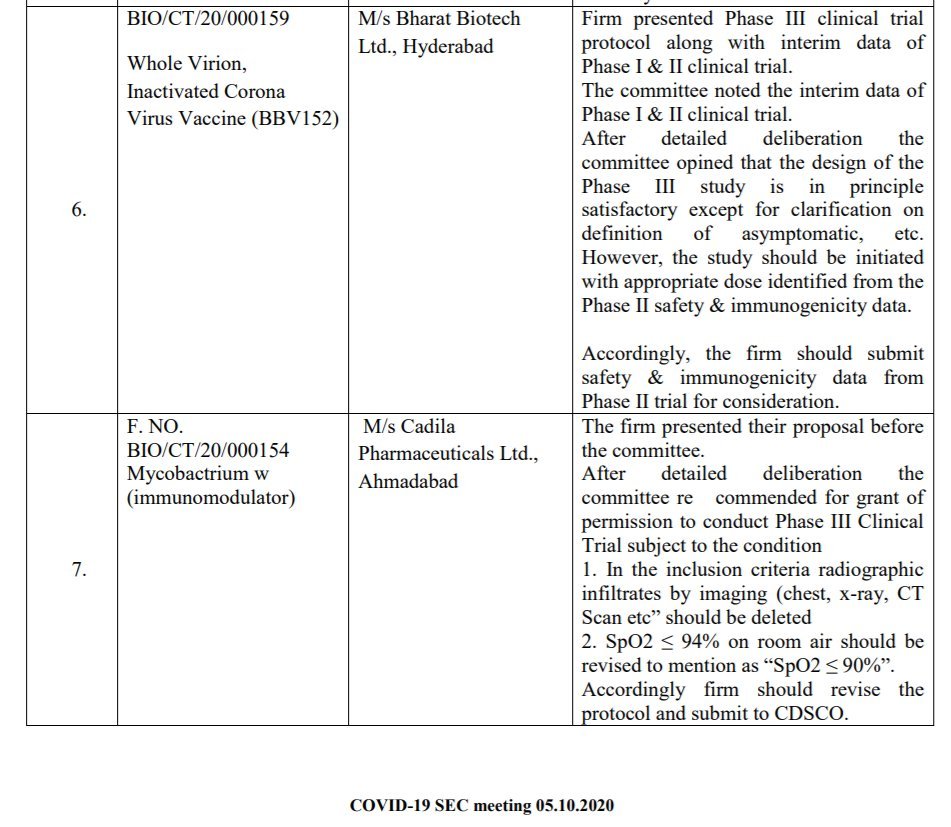
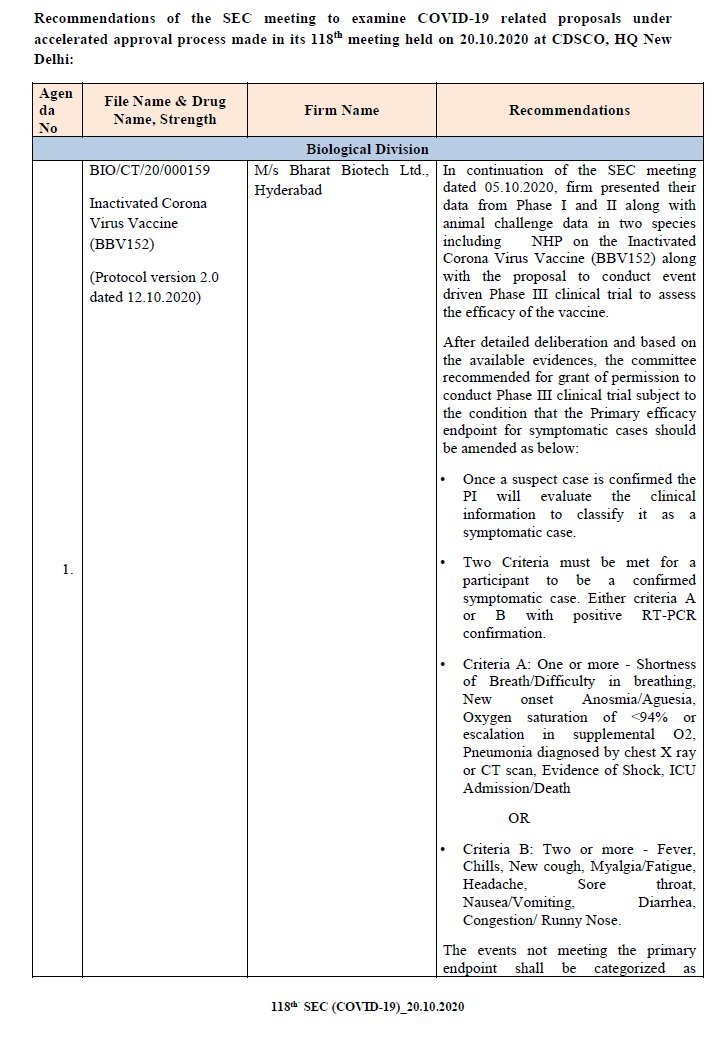
 COVAXIN by @BharatBiotech: Firm finally submitted Phase I, II & complete animal challenge study data to CDSCO. There was CHANGE in dose strength in mid of Phase II. To conduct Phase III.
COVAXIN by @BharatBiotech: Firm finally submitted Phase I, II & complete animal challenge study data to CDSCO. There was CHANGE in dose strength in mid of Phase II. To conduct Phase III. @nuts2406 @prat1112001 @pash22 @RPrasad12 @MenonBioPhysics
https://twitter.com/das_seed/status/1319310349330534400
 Phase III of COVAXIN initiated in some clinical trial sites.
Phase III of COVAXIN initiated in some clinical trial sites. Image via @ProfTariqManso1. Trial volunteers to receive travel expenses and other benefits as per ICMR guidelines.
@malini_aisola @pash22 @giridar100 @amarjesani @RemaNagarajan
https://twitter.com/das_seed/status/1322553962411732993

 Read on Twitter
Read on Twitter