Second thread on $FGEN roxadustat in NDD.
First thread here: https://twitter.com/Biomaven/status/1304433750911188993?s=20
First thread here: https://twitter.com/Biomaven/status/1304433750911188993?s=20
So $FGEN stock price was basically unchanged w/ $AKBA's failure, even though this likely removed a significant potential competitor in NDD - analysts gave vada maybe a 25% market share. The reason for this appears to be whether the vada failure reads into roxa approval/labelling.
Now FDA is obviously wary of this space given the historical issues with ESAs - that's why FGEN was told to compare vs. placebo if they wanted to avoid a black box. That was always going to be a difficult trial given more placebo pts were expected to drop out and in fact did so
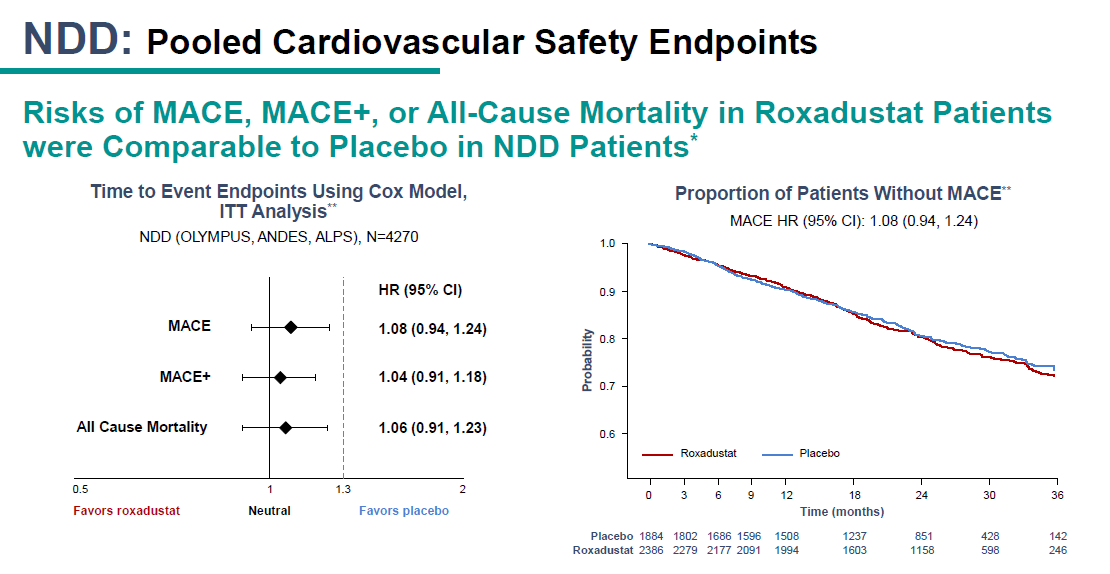
That means the statistical analysis of the trials becomes hard, as you face the issue of placebo pts dropping out and then having a MACE event. So has to be an ITT analysis based on time to event. Further, as more pt years on roxa, have to exposure adjust AEs.
We still don't have full data from these NDD trials - hopefully ASN & publications will fill in the details. But we do have topline data showing roxa met non-inferiority margin set by EMA and now apparently agreed to by FDA. Note roxa did trend slightly worse than placebo here
Could be noise, could be real, or could be not all MACE events from placebo dropouts were captured. We may know more with detailed data.
But either way, there is nothing in this disclosed data to justify a black box warning. And that doesn't change in the slightest w/ AKBA data.
But either way, there is nothing in this disclosed data to justify a black box warning. And that doesn't change in the slightest w/ AKBA data.
Where does NDA process stand? We are 100 days from PDUFA date, and at quarterly call a month or so back company said no AdCom yet, and interactions w/ FDA were going well, with labelling discussions "up next." At call this week, reiterated FDA interactions going smoothly.
So my own read is no AdCom, and hence no Black Box - I really do *not* believe the FDA would require a Black Box based on this data without a comprehensive AdCom discussion. So my read from no AdCom is also no Black Box, and also modest chance of approval prior to PDUFA date.

 Read on Twitter
Read on Twitter