Our latest preprint, from Anna McDermott & @EmilyEastburn1 in the lab, is live today!
"Effects of chondrogenic priming duration on mechanoregulation of engineered cartilage anlagen"
https://www.biorxiv.org/content/10.1101/2020.09.02.280115v1
A paper thread:
"Effects of chondrogenic priming duration on mechanoregulation of engineered cartilage anlagen"
https://www.biorxiv.org/content/10.1101/2020.09.02.280115v1
A paper thread:
Another step forward in our long chase for the answer to which cells are responsible for sensing mechanical forces during bone regeneration, this is a wonderful collab w/ @dannykelly1978
(with whom I wrote my very 1st grant as a PI!)
(with whom I wrote my very 1st grant as a PI!)
A side word of advice for my @NewPI_Slack friends: finding generous collaborators like @dannykelly1978 early in your faculty career can change your life.
But funding mechanisms that support true international collaborations are incredibly difficult to find.
This project was uniquely enabled by the @NaughtonFellows program, which allowed Anna to spend 2 years of her PhD in Dublin with Danny @tcdTCBE.
This project was uniquely enabled by the @NaughtonFellows program, which allowed Anna to spend 2 years of her PhD in Dublin with Danny @tcdTCBE.
The question of this paper started for me a decade ago, when I was a PhD student in @guldberg_bob's lab.
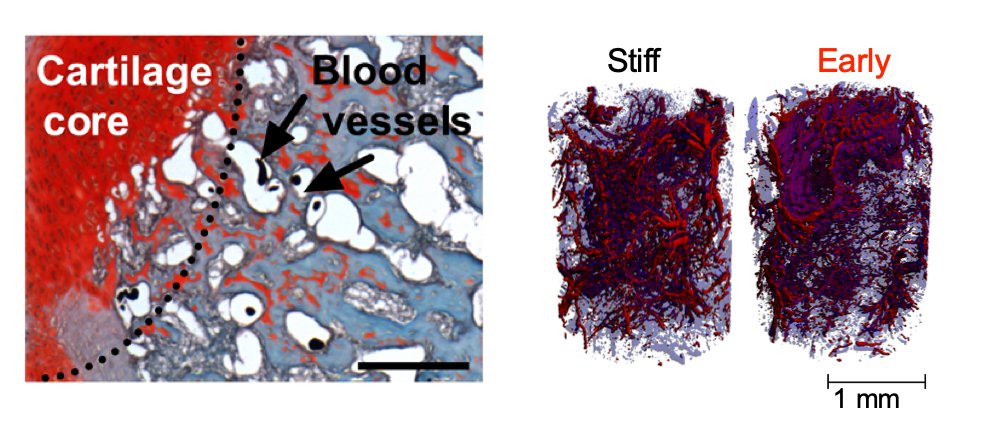
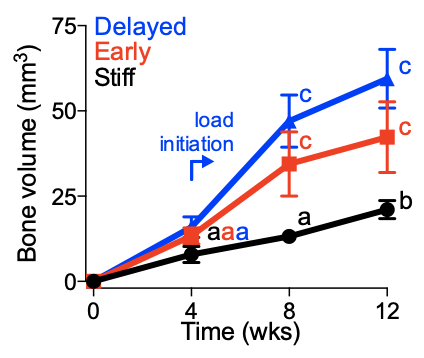
We found that applying mechanical forces to large bone defects promoted endochondral bone regeneration and dramatically altered angiogenesis. https://www.pnas.org/content/108/37/E674
We found that applying mechanical forces to large bone defects promoted endochondral bone regeneration and dramatically altered angiogenesis. https://www.pnas.org/content/108/37/E674
This raised new questions: who is responsible for sensing and responding to these mechanical cues and why does loading promote endochondral ossification (ie, bone formation through a cartilage intermediate)? Is it the osteo/chondroprogenitor cells? Or is it the endothelial cells?
Together with @sherberg128, @dannykelly1978 and @tissue_engineer, etc., Anna's first paper developed an approach that would allow us to specifically promote endochondral regeneration by engineering the cartilage anlage: https://stm.sciencemag.org/content/11/495/eaav7756
As we'd seen in the @PNASNews paper, loading regulated both chondrogenesis and angiogenesis during development-mimetic regeneration.
As it happens, @EmilyEastburn1 worked as an undergrad with @m_ruehle in the @WillettLabs on the latter question:
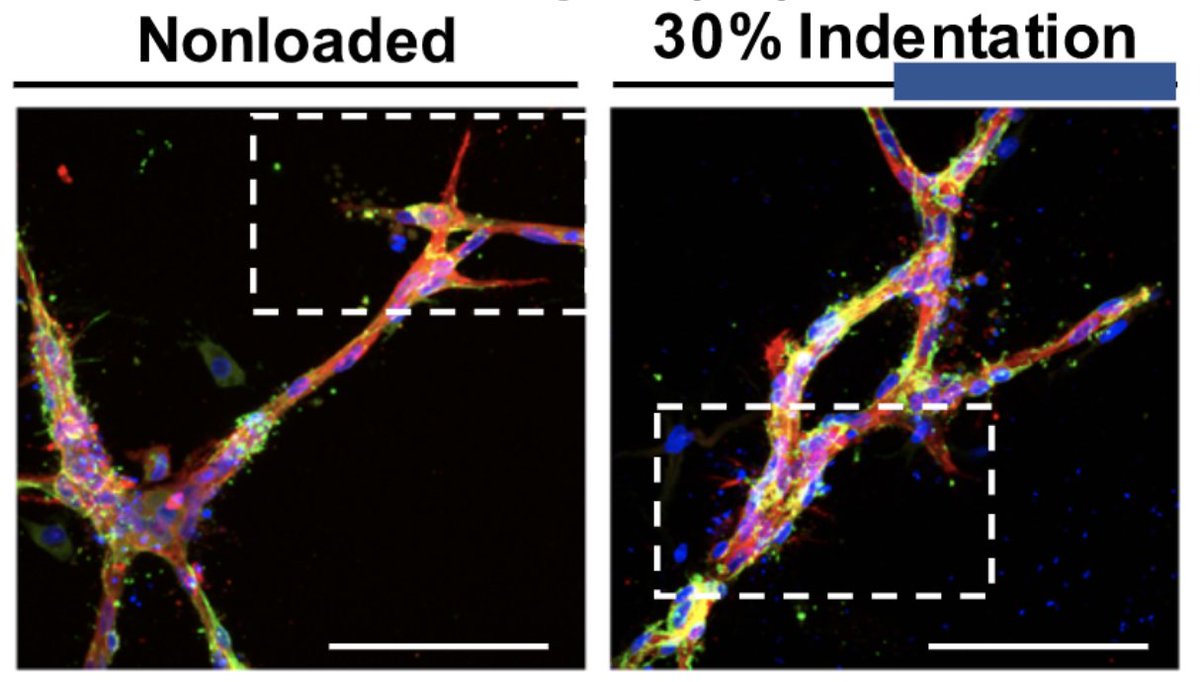
Do mechanical forces directly regulate new blood vessel formation?
https://advances.sciencemag.org/content/6/34/eabb6351.abstract
This one was special, as I told here: https://twitter.com/jboerckel/status/1296984068261896192?s=20
Do mechanical forces directly regulate new blood vessel formation?
https://advances.sciencemag.org/content/6/34/eabb6351.abstract
This one was special, as I told here: https://twitter.com/jboerckel/status/1296984068261896192?s=20
But we still need to understand how loading affects the chondrogenic part of endochondral ossification.
We found in the @ScienceTM paper that bone formation was particularly enhanced when loading was initiated at the stage of chondrocyte hypertrophy.
We found in the @ScienceTM paper that bone formation was particularly enhanced when loading was initiated at the stage of chondrocyte hypertrophy.
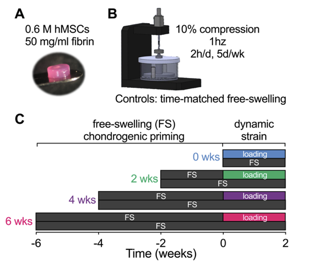
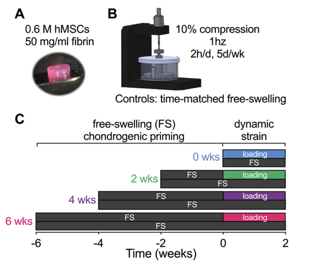
Therefore, we designed this study with @dannykelly1978 to determine how chondrocytes, at different stages of endochondral maturation, respond to dynamic mechanical compression in a bioreactor:
https://www.biorxiv.org/content/10.1101/2020.09.02.280115v1
https://www.biorxiv.org/content/10.1101/2020.09.02.280115v1
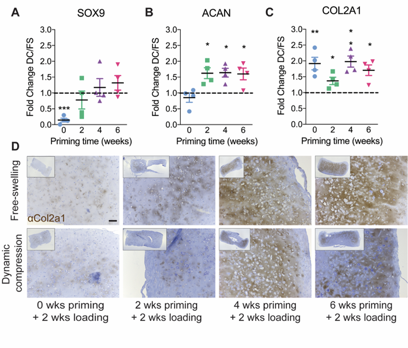
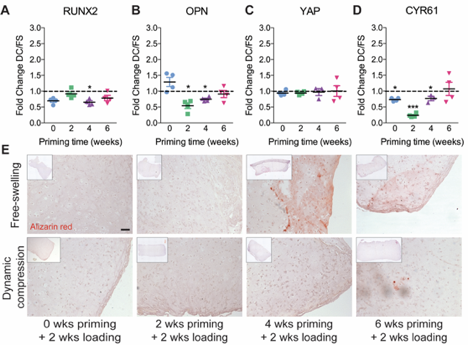
We found that loading regulated chondrogenic gene expression, in a manner dependent on the timing of load initiation, and altered the distribution of matrix deposition.
The timing of load initiation also determined whether loading was inhibitory (early) or permissive (late) of mineral deposition.
Taking all these findings together, we conclude that the effects of mechanical loading depend on the timing of when the load is applied, and coordinately control both osteo/chondro-progenitor lineage commitment and new blood vessel formation.
These findings have important implications for the design of fixation devices that control bone stability during injury repair and the development of physical therapy regimens that will promote rather than impair tissue regeneration.
Keep an eye out for further work from Anna in this space, as she's currently a postdoc with the one and only Nick @WillettLabs, who's leading the field of Regenerative Rehabilitation to new heights!

 Read on Twitter
Read on Twitter