Astronomers used Hubble to look at the Moon during an eclipse to discover life on Earth.
Well, kinda. https://www.syfy.com/syfywire/astronomers-use-hubble-during-an-eclipse-to-detect-life-on-earth
Well, kinda. https://www.syfy.com/syfywire/astronomers-use-hubble-during-an-eclipse-to-detect-life-on-earth
2/ During a lunar eclipse, the Earth blocks the Sun from the Moon. So if you're standing on the Moon you see the Earth sliding into the Sun's DMs^h^h^h^h across the face of the Sun.
3/ That means sunlight passes through Earth's atmosphere on its way to the Moon. Molecules like ozone absorb specific colors of light, so by looking at the Moon you can detect those molecules in Earth's atmosphere.
More info on that:
More info on that:
4/ Ozone absorbs some light in the blue part of the spectrum and some in the ultraviolet. UV light from space doesn't make it to the ground, so astronomers used Hubble to observe the Moon during the January 2019 eclipse to look for this absorption.
5/ Remember that eclipse? It was the one where an asteroid the size of a beachball slammed into the Moon at 60,000 kph! Sadly, Hubble wasn't looking at that part of the lunar surface. Too bad. That would've been an *amazing* bonus. https://www.syfy.com/syfywire/update-the-lunar-eclipse-impact-was-from-a-beachball-sized-rock-moving-at-61000-kph
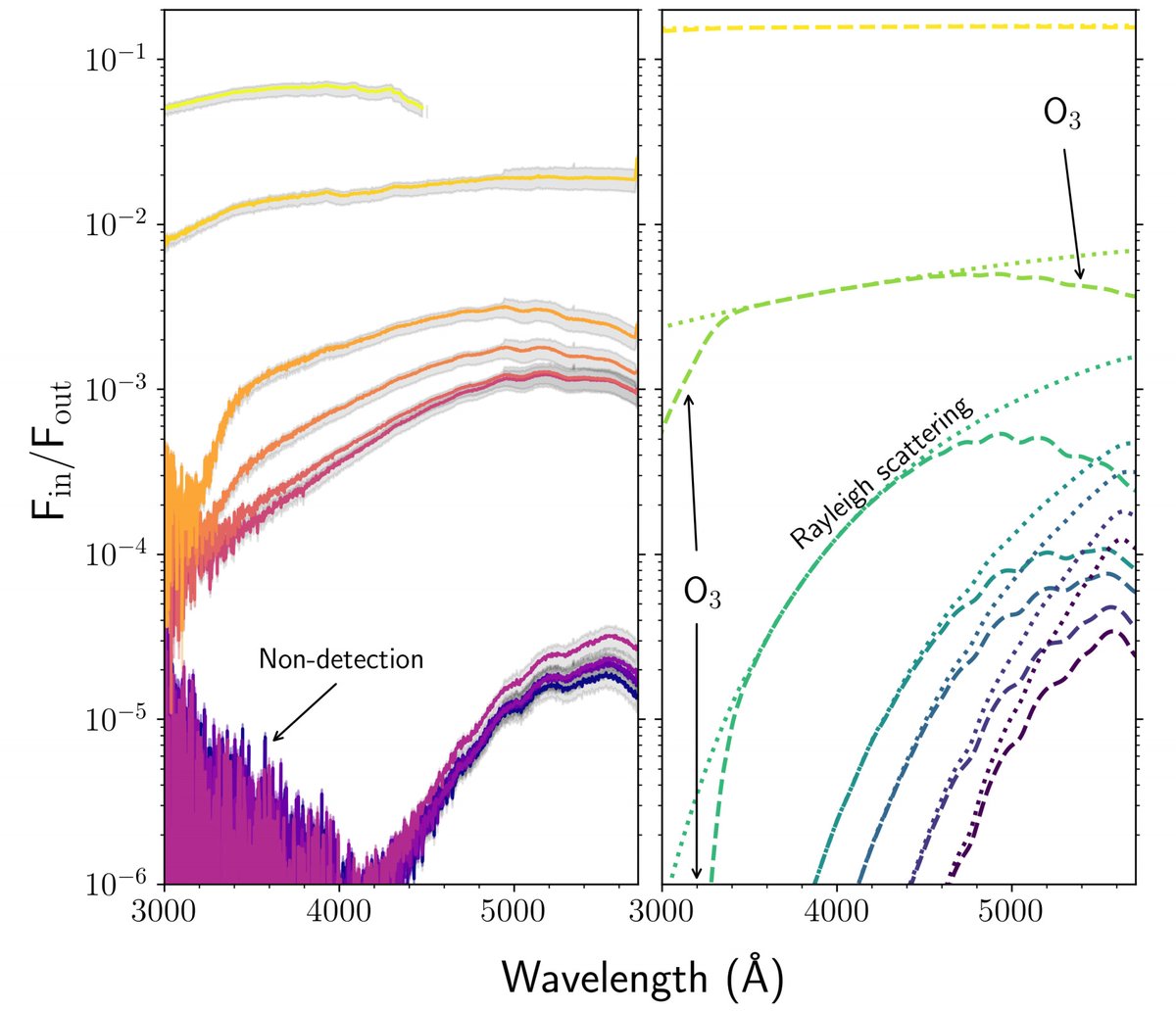
6/ The observations were extremely tricky, but in the end they got it! They saw the dip in light in blue and UV indicating the presence of ozone in our air. The observations are on the left in this plot, and a physical model of what they expected on the right...
7/ … I know it's confusing. But, if there were *no* ozone, the orange line on the left would be flatter. But it dips down at both ends due to ozone absorption (the model on the right shows that; the dotted line would be if there were no ozone, and the dashed line if there is).
8/ So they detected ozone, and that's created by life. Oxygen gets hit by UV light and forms ozone, so in a sense they really did detect life on Earth.
So why do this? Because we do something very similar with planets around other stars.
So why do this? Because we do something very similar with planets around other stars.
9/ We see starlight passing through their atmospheres and we can in principle determine what molecules are there. It's pretty hard to do, but this shows it can be done in the UV, which is new. We'll need WAY bigger 'scopes, but the technique works.
10/ I am also legally obligated to mention that these observations were done with the STIS camera on Hubble, which I worked on for many years. I *LOVE* seeing it get used to do innovative stuff like this. https://www.syfy.com/syfywire/why-im-watching-hubble-repairs-so-intently
11/11 Thirty years ago it would've been nuts to think we could routinely detect other planets, but now that's true. In 30 more we may be routinely observing their air, seeing what's in it. Incredible.
https://www.syfy.com/syfywire/astronomers-use-hubble-during-an-eclipse-to-detect-life-on-earth
/fin
https://www.syfy.com/syfywire/astronomers-use-hubble-during-an-eclipse-to-detect-life-on-earth
/fin

 Read on Twitter
Read on Twitter