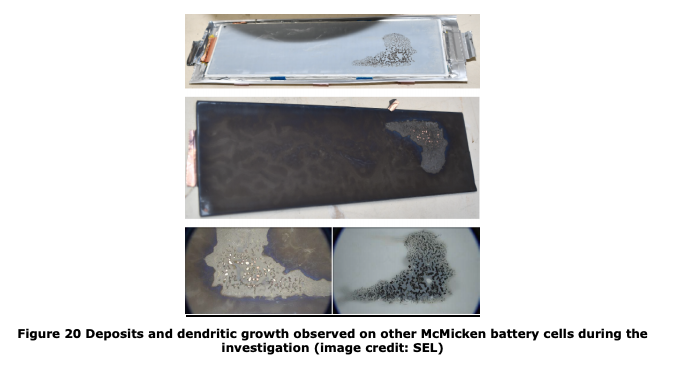
DNV-GL report on APS battery fire. Hypothesis that it was an actual lithium trigger that started runaway in the first cell. This much Li metal deposition in this type of cell at C/3 rates at ~25˚C to 30˚C should be _very_ hard to do. 1/n https://www.aps.com/-/media/APS/APSCOM-PDFs/About/Our-Company/Newsroom/McMickenFinalTechnicalReport.ashx?la=en&hash=50335FB5098D9858BFD276C40FA54FCE
That is a crazy amount of Li in that picture. Over the last few years we've tested and abused 1000(? likely more) cells and the only way we've seen this much lithium at currents this unit saw were if the cells were too _cold_ and charged too quickly. http://dx.doi.org/10.1149/1945-7111/ab6c56 2/n
The folks at DNV-GL really know what they're doing and finding this metallized cell is fantastic. This graph in particular is really well written: it bound that the presence of lithium is the root cause for a _number_ of different failure modes. 3/n
They go on to suggest one mechanisms that Li filamentary shorts crossing over created a runaway condition. But! This isn't the only route to runaway. In fact, _most Li_ filamentary shorts fizzle out because the Li is thin and oxidizes before the cell can go into runaway. 4/n
This is where this safety diagnosis gets really hard, b/c the presence of Li alone creates a world of problems. We have seen 100's (maybe 1000's) of cases where these Li shorts do _not_ lead to runaway. In fact, we've not been able to cause a Li filamentary runaway in my lab. 5/n
Not to say it can't happen! This is a big cell, and we're talking a 1 in 100,000 type event even with this much Li. But a mechanism we have seen that is more likely to cause catastrophic damage is indicated in that paper ( http://dx.doi.org/10.1149/1945-7111/ab6c56). 6/n
If too much Li builds up, the cell has no "reservoir" to store it. These cells are like phyllo pastry, but even thinner. The electrodes are ~50 µm each, and the separator is < 20µm, over an area that is >> 100cm^2, with > 30 stacks of electrodes per cell. So lots of layers. 7/n
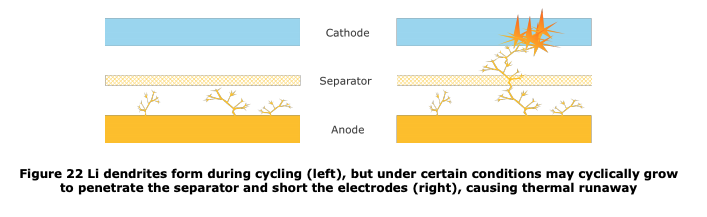
The amount of Li down in figure 20 is at least ~3-5µm, likely more, pushing into a sorta pliable separator of 20µm. My biggest nitpick of the survey is Figure 22. This is not to scale. There's _no_ space between the electrodes and the separator, let alone the ocean depicted. 8/n
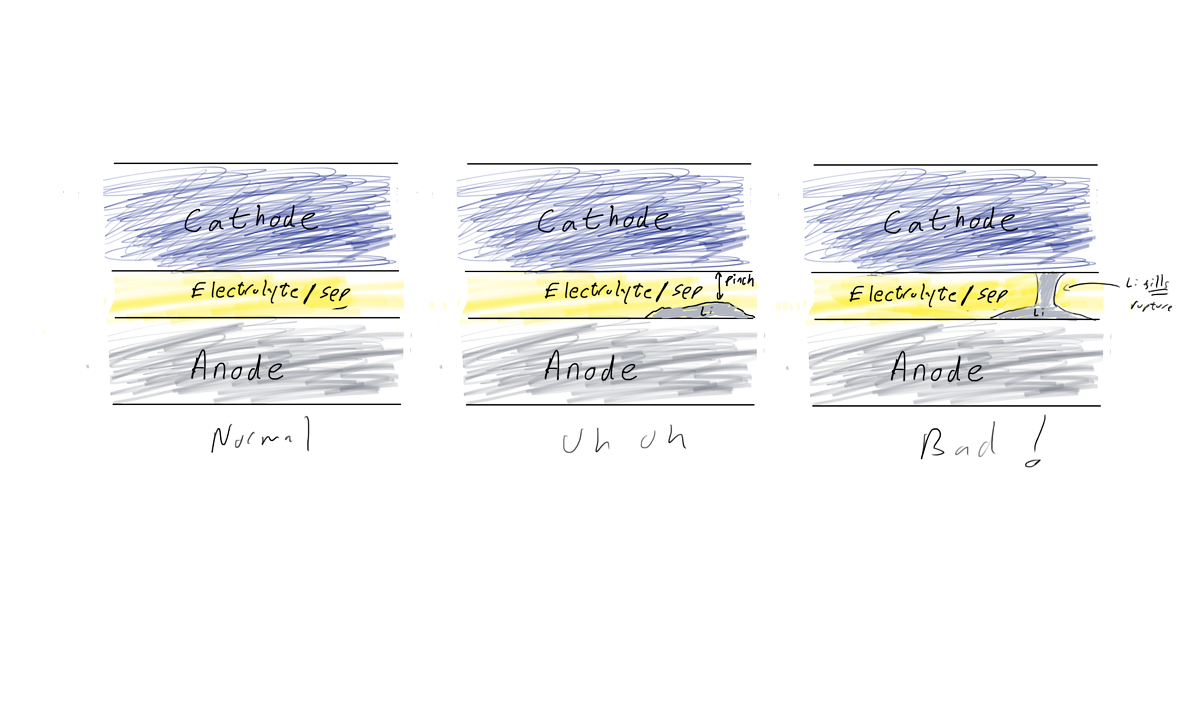
I'd go with this slightly different sketch. a) the separator is not floating between the electrodes b) the lithium is represented by real mass (per figure 20), not just fingers (per figure 22). 9/n
This matters because this undesigned mass is now undesigned _volume_ & may create a hard short that can't be oxidized away. Or this mass will create pressurized gas when it decomposes with the electrolyte and can lead to other parts of the cell pinch shorting to compensate. 10/n
But the language used in the report fully encompasses all of these complex possibilities, and that is what is important. The big point is that the cell was never designed to have this much Li, and this uncertainty around this volatility in a constrained volume is dangerous. 11/n
The downstream analysis is sobering. One cell went into thermal runaway (not even sure it was a fire), and a non-trivial HVAC and fire suppression system could not outpace the propagation. This is _active equipment_ that increases the $/kWh and decreases the kWh/kg 12/n
This is a _very_ important report. Containment adds expense and is not perfect. We need to better understand the link between internal structural events, short circuit formation, and thermal runaway to 1) decrease cost 2) increase safety and 3) increase energy density 13/n
Four firefighters were injured in this incident, but I think (hope) they are all OK now. The careful analysis and bounding by DNV-GL and APS will help us better bound systems and cells going forward. 14/n https://www.azfamily.com/news/injured-firefighters-identified-from-substation-battery-explosion-in-surprise/article_c28a7f1e-631c-11e9-9bf1-bfa00f273619.html
Big takeaways for me? a) this much lithium should never be present in a thermally conditioned lithium ion cell. This is really strange. b) downstream containment/mitigation is important, but is expensive and not a panacea 15/n
_Finally_ c) shameless plug/drinking my own koolaid, we need better understanding of battery damage events so we can create failsafe cells. cells can be designed to gobble short circuits and live to tell the story. designing for failure i important https://doi.org/10.1039/C6EE02782B n/n
@wesleykchang points out charge cycles at 0.8C, not 0.3C. So shameless plug d) longer duration systems that require lower power density _might_ lead to safer systems, if proper headroom on power is kept. Still, 0.8C at ~25˚C should not lead to this much Li n+1/n.
important correction: @TheEnergyNerd pointed out these are for frequency regulation (I assumed b/c AZ there were 4 hour cells and didn't read front matter well). So these, by design, should be designed for 1 C cycles, and the Li deposition is still very odd/not good. n+2/n
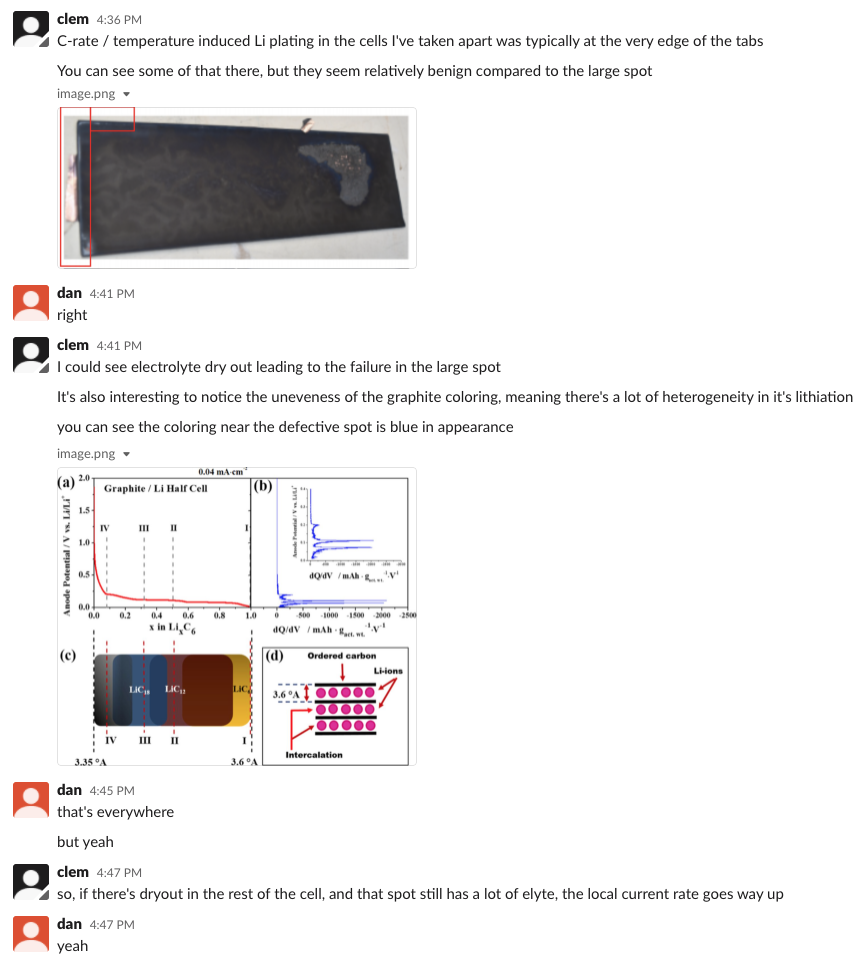
my group lives for this analysis btw: alum @CBommier chimes in via slack with the receipts. Note the coloration changes in figure 20. Could be lithiation variation, could be electrolyte dry out patterns. My friend and former colleague Craig Arnold should be all over this. n+3/n

 Read on Twitter
Read on Twitter