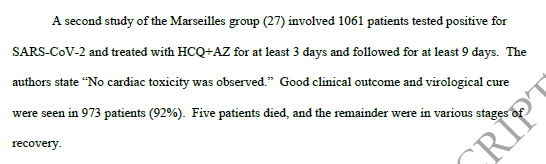A thread.
I was alerted to a Newsweek paper by Yale professor of Epidemiology Harvey Risch in which he argues quite persuasively that #Hydroxychloroquin, #HCQ (= Azithromycin + Zinc) is an effective treatment for early-stage #Covid19 and has been opposed on spurious grounds
I was alerted to a Newsweek paper by Yale professor of Epidemiology Harvey Risch in which he argues quite persuasively that #Hydroxychloroquin, #HCQ (= Azithromycin + Zinc) is an effective treatment for early-stage #Covid19 and has been opposed on spurious grounds
Here's a link to the Newsweek article. It refers to a paper by Risch in the Americal Journal of Epidemiology ( #AJE) which details the evidence. https://www.newsweek.com/key-defeating-covid-19-already-exists-we-need-start-using-it-opinion-1519535
I looked at the #AJE paper. The abstract is here as a screen shot. It is freely downloadable as a pdf and there is a supplemental 'doc file with the details of 5 studies.
The bit in the abstract I highlighted says:
"Five studies, including two controlled clinical trials, have demonstrated significant major outpatient treatment efficacy" I looked at the Supplementary data file to which there is this link at the bottom of the web page:
"Five studies, including two controlled clinical trials, have demonstrated significant major outpatient treatment efficacy" I looked at the Supplementary data file to which there is this link at the bottom of the web page:
The full link to the web page of the abstract is here: https://academic.oup.com/aje/article/doi/10.1093/aje/kwaa093/5847586. You can track it down and download the supplementary data file. I did just that and what I get is:
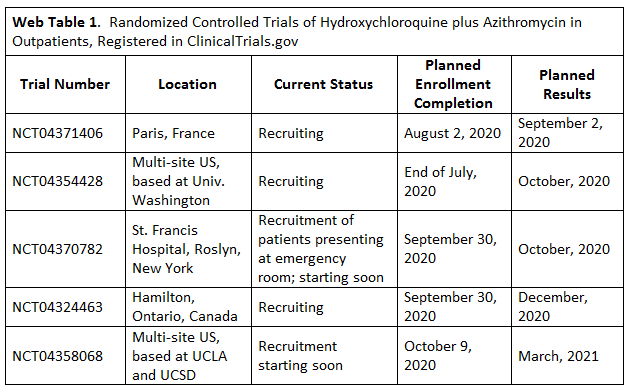
A table in Word detailing the 5 clinical trials of #HCQ. The interesting thing is that NONE of these trials have yet reported. Recruitment ends later this year for all 5, 4 plan to have results in 2020 and the last one in March 2021.
Clearly, these cannot be the 5 studies that Risch refers to in the abstract. Let's look at the pdf of the full paper; That should give us the details of the 5 papers that give the evidence on which to make such a strong Statement in the abstract.
It turns out that the pdf link is not to the paper as it appears in #AJE but a pdf of the original manuscript as submitted. Maybe that is as good, just that I was expecting something different. Any way this full paper refers to the 5 RCTs I mentioned earlier. It also describes..
Several other studies but not in the usual Meta-analysis tabular format. He argues that trials of single-agent HCQ cannot be generalised to HCQ+AZ/Doxycycline. he refers us to:
a) a French non-randomised but controlled trial of 42 pts showed a 50-fold benefit!!!
a) a French non-randomised but controlled trial of 42 pts showed a 50-fold benefit!!!
But it is not clear what the outcome was or how big the control group was. The benefit was only 25-fold in patients with lower Resp tract infection. So in an n=42 trial, we are into sub-group analysis; Duh!
Risch answers the "But-It-is-not-randomised!" criticism by praying in aid the huge (25- or 50- fold) magnitude of benefit. If the benfit was just say 2 or 3 fold then he might have conceded the point that it was a Type-1 error due to differences in the 2 groups.
This is to miss the point that randomisation is not done to achieve near-identical groups. its purpose is to eliminate bias due to unknown factors. He answers the small sample size criticism with a school boy howler:
He would have you believe that you need large sample sizes only to avoid the risk of missing a true effect, but when the effect size is so huge - 50-fold (Im still not clear what the outcome was, was it death?) then sample size ceases to matter. This is like the #Itoluzimab trial
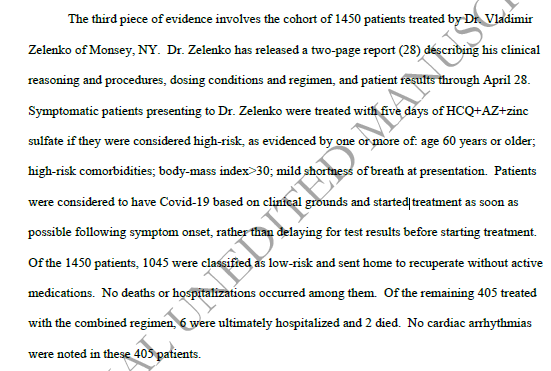
The 3rd study is this one: Not a trial but one doctor's experience of a cohort of patients he treated - a 2 page report. Brilliant!
The 4th study Risch quotes is from Brazil. Patients who declined HCQ + AZ formed the control group. the outcome was need for hospitalisation.
The results of this self-selecting controlled study are here:
1.2% of those treated < 7 days vs 3.2% (after 7 days) v 5.4% (control group) needed hospitalisation. Biases, anyone?
1.2% of those treated < 7 days vs 3.2% (after 7 days) v 5.4% (control group) needed hospitalisation. Biases, anyone?
The rest of the p[aper is aimed at arguing that in short term use#HCQ is safe and estimates of side-effects must not be extrapolated from studies with high doses for long durations in other serious conditions. Fair point.
Conclusion: I think the claims are premature.
If there was reasonable ground to believe thatHCQ+AZ hold promise we really should have done 1 proper good RCT on the #RECOVERY #dexamethasone model and settles the question.
If there was reasonable ground to believe thatHCQ+AZ hold promise we really should have done 1 proper good RCT on the #RECOVERY #dexamethasone model and settles the question.
But hopes have been raised and it'll be hard to deny eager patients the drug at an early stage. It is probably not unsafe at all and may do some patients some good. But predictions that it would have saved thousands of deaths seem far fetched.
END
END

 Read on Twitter
Read on Twitter