Much of the arguments about #HCQ for COVID_19 either for or against have been based on personal convictions and biases than reliable data. We have heard conspiracy theorists claim that #HCQ arms in large clinical trials were deliberately set up to fail. Thread
Much of the criticism for previous randomized trials ( #RECOVERY and #SOLIDARITY) have had to do with the fact that #HCQ was tested at the “wrong time” (moderate to severe disease) and the “wrong dose” (too high).
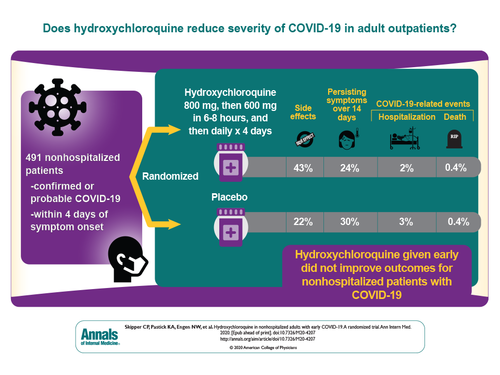
We now have a study that tests #HCQ in early #COVID_19 (outpatients, symptomatic lab-confirmed/ probable COVID_19) with lower doses (600 mg). Randomized, double-blind, placebo-controlled, 491 patients in the US & Canada. Primary endpoint - change in symptom severity over 14 days
This is not a perfect study. Only 58% had testing for SARSCoV-2 due to shortages of testing in the US. The sample size wasn’t large enough to detect smaller differences in outcome. Only 423 contributed to the primary endpoint data. Yet, this is an important study because…
the study addresses much of the criticism against other #HCQ studies. #HCQ was started early in the course of the disease. Doses were not too high. It was randomized, allocation concealed, double-blind and placebo-controlled, thereby eliminating bias.
The study showed no differences in symptom severity, hospitalization or mortality. This study did not find any benefit of #HCQ in outpatients with early, mild #COVID_19
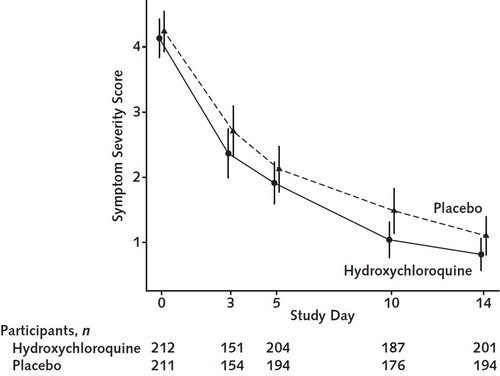
I will end with this figure from the paper. Symptom severity over 14 days. If this had been a single arm #HCQ (or the placebo!) study, many would have claimed the dramatic reduction in symptoms as a breakthrough. Just shows why we need valid controls. Every. Single. Time.

 Read on Twitter
Read on Twitter