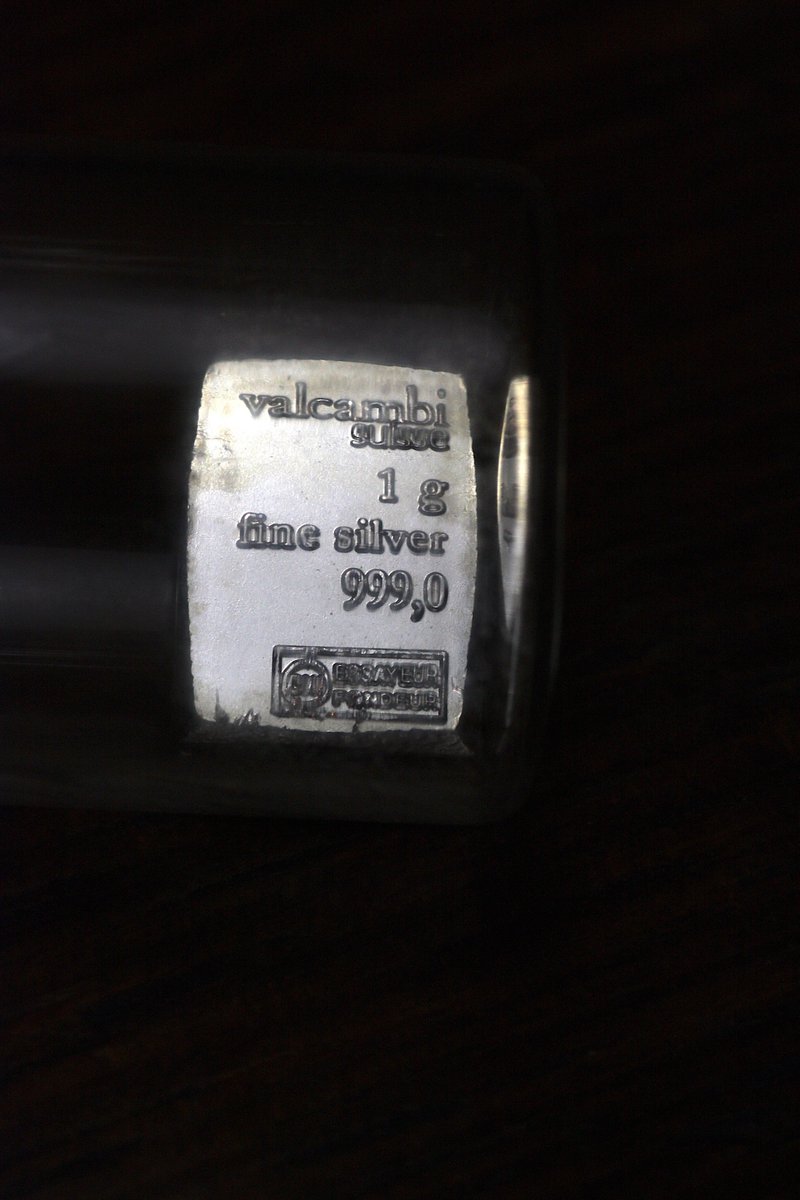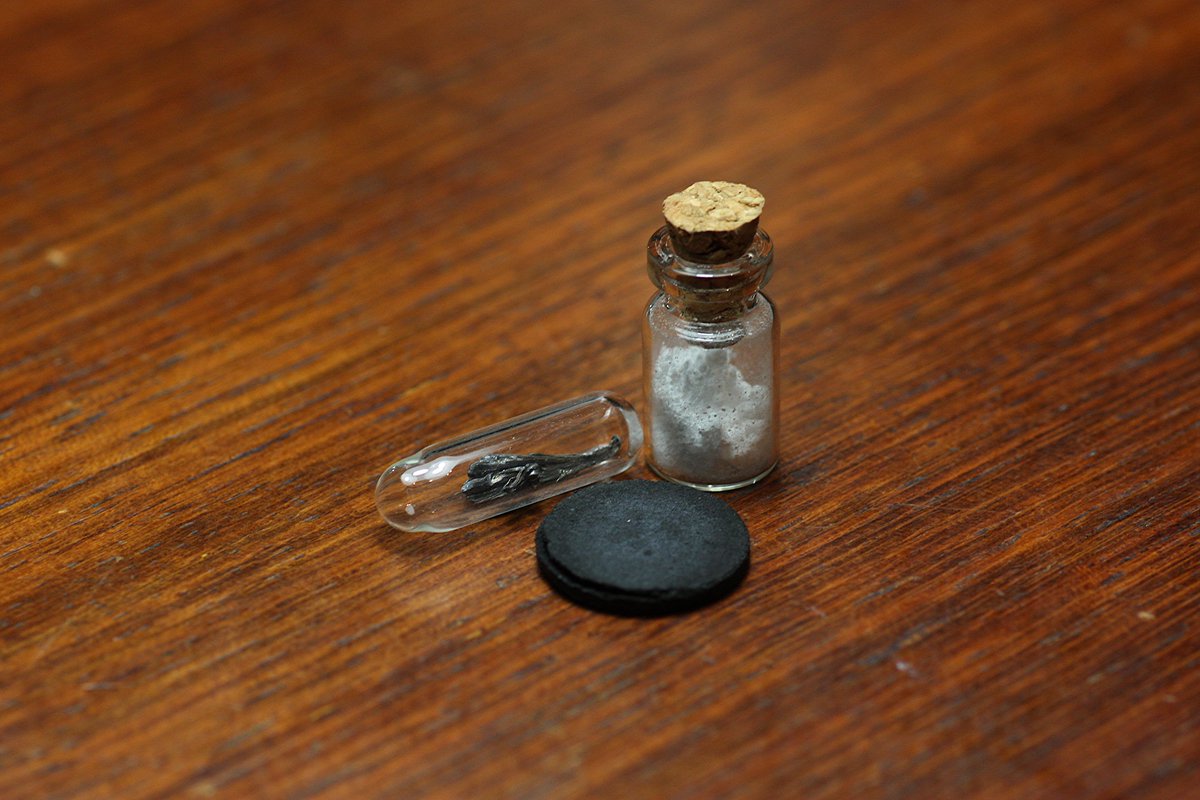For no other reason than lockdown cabin fever, here's some #elementphotos from my various element collections, one element at a time. Here's the 3 full sets; the wall set's finished, the 'tray' set is almost done, and the briefcase is not far behind. #elementsets #IYPT2019
Here's my wider collection. Completing the tray set is now only waiting on a cesium sample that's been waiting for a plane at San Francisco airport for 2 weeks. Grrrr. Tray set tries to have both element sample and something else that contains that element. #elementphotos
To start the #elementphotos off, here's three isotopes of hydrogen. Hydrogen and deuterium are low pressure ampoules that glow in an electric field (e.g. tesla coil, plasma ball). Tritium is radioactive and used in long-life glow sticks (the glow is an applied phosphor).
Here's a helium discharge tube with an electric field applied. Larger ampoule than the hydrogen ones, but otherwise the same. #elementphotos
I have a set of these larger discharge tubes for all the elemental gases (except F - far too reactive). Great for talking to school kids about the periodic table.
More #elementphotos to come sporadically over the next few days/weeks as I get time to take some nice photos.
More #elementphotos to come sporadically over the next few days/weeks as I get time to take some nice photos.
Here's Lithium metal and its carbonate salt (Li2CO3). The pure metal is air/moisture sensitive, so is contained in an argon-filled ampoule. #elementphotos
Pic 1: Two pieces of Beryllium, along with a crystal of Morganite, which is a form of beryl (Be3Al2Si6O18) with Mn(II) impurities giving it a pink colour. A green emerald is essentially the same, but with Cr or V impurities. Pic 2: Be sphere. #elementphotos
Some Boron #elementphotos. The white powder is borax (Na2B4O7.10H2O).
Some bonus pics of the nice boron piece in the round container. Like shiny little black asteroids, but difficult to photograph. #elementphotos
OK, should have done this first. Here's the photos of the boron piece after a bit of photomanipulation, which shows it off a bit better. #elementphotos
Carbon #elementphotos. Pic 1: Large lump of natural graphite, plus industrial diamonds. Pic 2: Glassy carbon lump, diamonds, graphite powder, calcite (CaCO3) crystal. Pic 3: diamond and graphite.
Bonus carbon polymorph #elementphotos. Pyrolitic C (pic 1 far right) floating on magnets. Circle at back is C nanotubes coated on Al to give a 'superblack' material. MWNT = multi-walled nanotubes. Still looking for some Lonsdaleite.
Nitrogen's turn. Pic 1: nitrogen discharge tube. Pic 2: nitrogen gas ampoule and a sample of what is sometimes called saltpetre (KNO3). #elementphotos
Oxygen @elementphotos. Discharge tube's quite difficult to get a good photo of - glow is quite faint and diffuse white, though purple at the source to the eye. The container has a smaller gas ampoule plus two synthetic rubies (Al2O3 with Cr impurities giving the colour).
Fluorine #elementphotos. Ampoule contains 33% F2 in He. Or at least it did at some stage; elemental fluorine is incredibly reactive so I'm rather sceptical about how much is left. Crystal is Fluorite (CaF2).
Here's one reason it's called Neon Lighting and not, say, Argon Lighting. Both the brightest and the prettiest of the gases in a discharge tube. #elementphotos
Sodium #elementphotos. Sodium is reactive so it needs to be stored in an argon-filled ampoule (Pic 1), or (less ideal but more practical for use) under oil. Last Pic has an ampoule surrounded by rock salt crystals (NaCl).
Magnesium #elementphotos. Dark green crystal is olivine ((Mg,Fe)2SiO4), white crystal is magnesite (MgCO3).
Aluminium #elementphotos. Blue mineral is sodalite (Na8(Al6Si6O24)Cl2.
Here's what you get if you pour molten aluminium into cold water! #elementphotos
Silicon #elementphotos. White crystals are quartz (SiO2).
A large and beautiful lump of silicon that I got from .... somewhere or other. #elementphotos.
Phosphorus #elementphotos. Green crystals are apatite (Ca5(PO4)3(F,Cl,OH)).
Sulfur #elementphotos. White crystals are Selenite (CaSO4.2H2O), which curiously contains no selenium.
Chlorine #elementphotos. Chlorine becomes a yellow liquid under high pressure; here a high-pressure ampoule lies on a bed of rocksalt (NaCl).
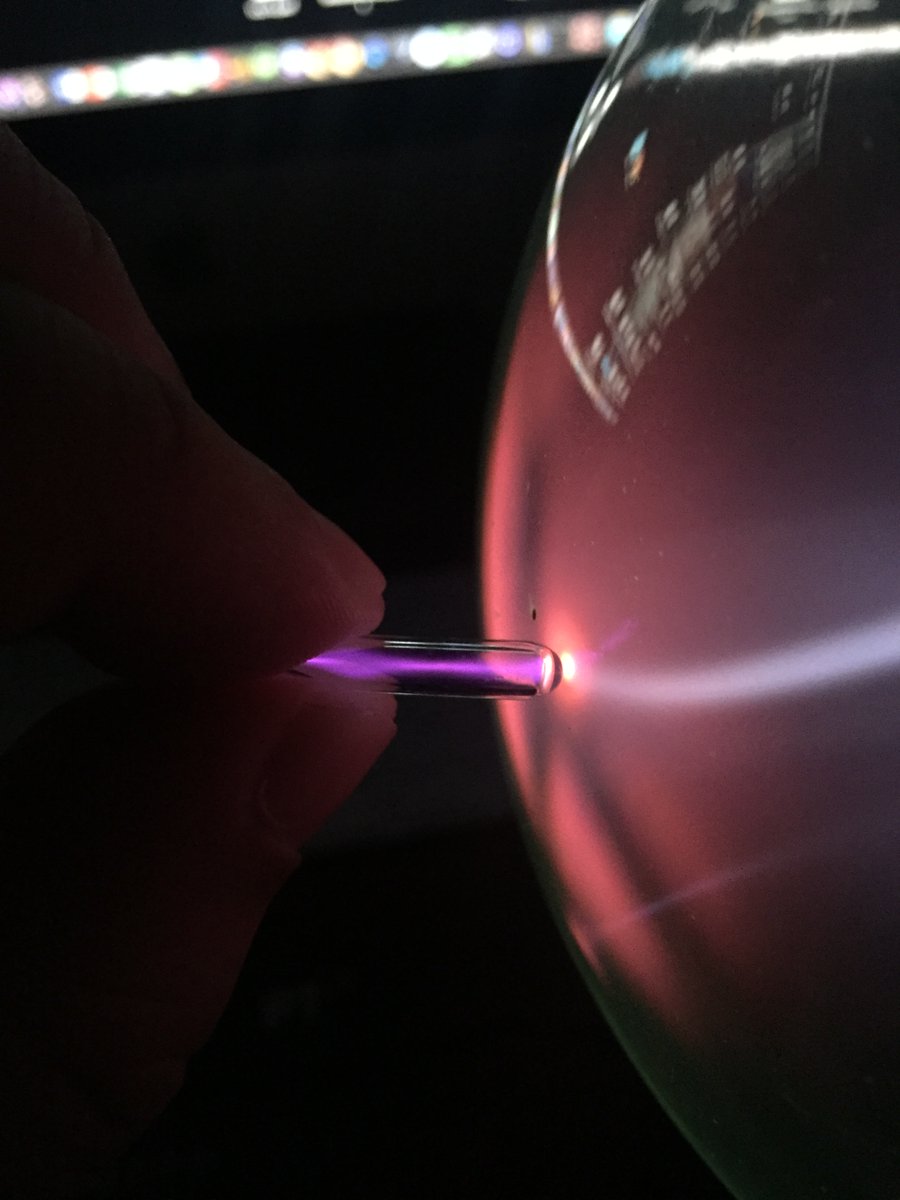
Argon #elementphotos. In the small low-pressure ampoule the discharge is quite a striking shade of purple, while in the larger ampoule it is a more diffuse pale blue, though purple at the point of contact with the ampoule tester.
Potassium #elementphotos. Potassium and moisture are not friends, so the best way to store it is in argon-filled ampoules. Storage under oil is more convenient but less successful at keeping it pristine (Pic 3). The white powder is KCl.
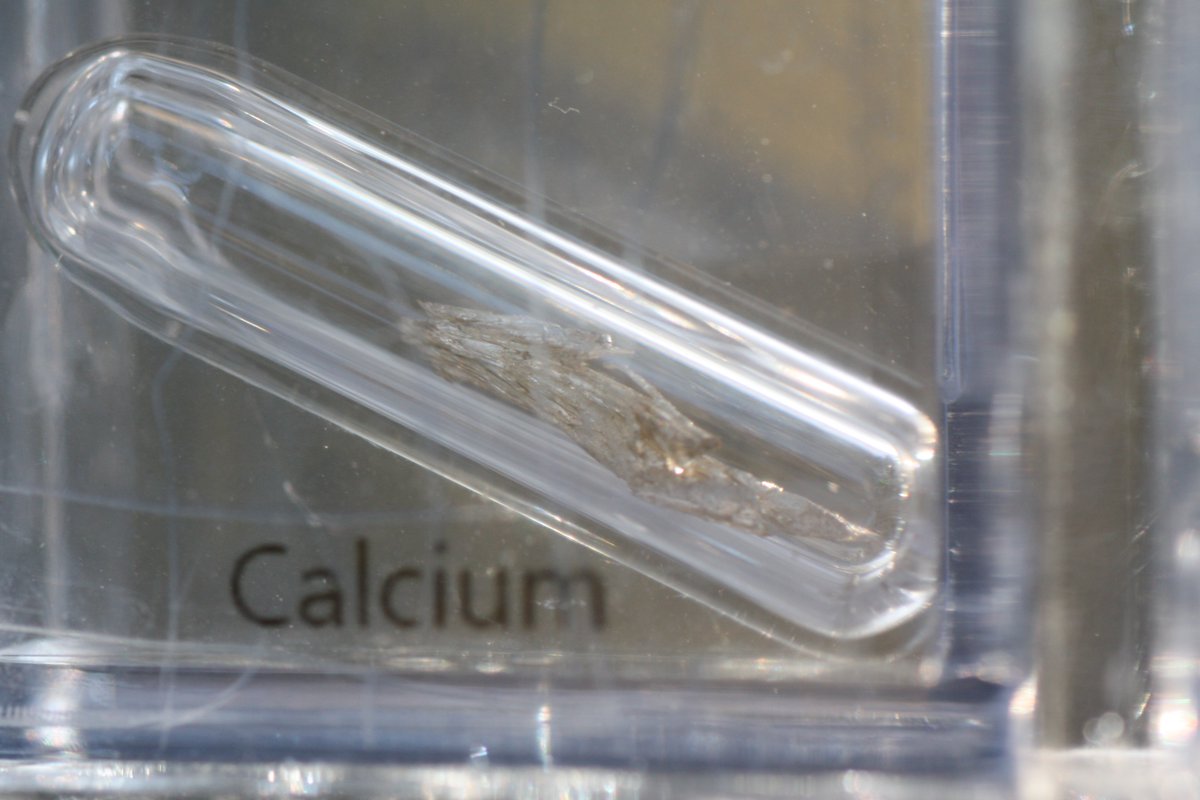
Calcium #elementphotos. Chalk, of course, is calcium carbonate (CaCO3).
Scandium #elementphotos - first of the transition metals. White powder is scandium acetate (Sc(O2CCH3)3.xH2O).
Titanium #elementphotos. Crystal is quartz containing streaks of rutile (TiO2).
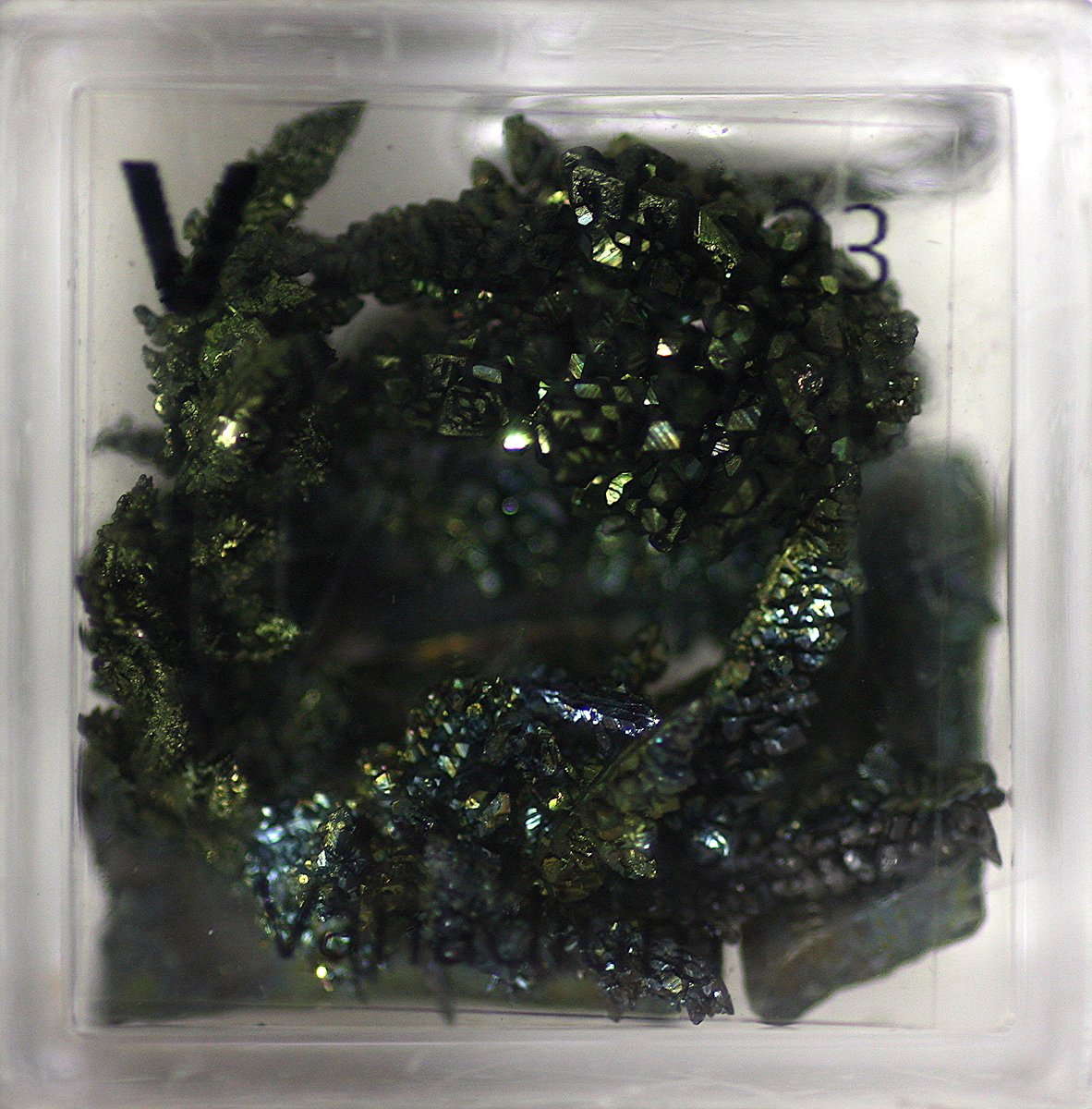
Vanadium #elementphotos. Blue compound is vanadyl sulphate (VOSO4.xH2O).
Chromium #elementphotos. Beautiful orange compound is potassium dichromate (K2Cr2O7).
Manganese #elementphotos. Mn oxidises really easily at elevated temperatures, so industrially it's usually made as heavily oxidised sheets (bubbled brown slabs). But unoxidised it's beautifully shiny. White powder is manganese sulphate (MnSO4.H2O).
Iron #elementphotos. Red powder is Hematite (Fe2O3), while the large crystals are Fool's Gold which, as the name suggests, contains no gold (iron pyrites, FeS2).
Cobalt #elementphotos. Dark mauve compound is cobalt chloride (CoCl2.6H2O).
Nickel #elementphotos. Coin is a relatively rare example of a pure nickel coin. Green compound is nickel sulphate (NiSO4.6H2O).
Copper #elementphotos. Like silver and gold, copper can be found naturally as nuggets. Blue compound is copper sulphate (CuSO4.5H2O).
Zinc #elementphotos. White compound is zinc oxide (ZnO), most of which is used to make rubber, but some of which is slathered on the noses of cricketers. @Aus_ScienceWeek #ScienceWeek
Gallium #elementphotos, including a gallium nitride (GaN) Blue LED. Unlike M&Ms, gallium will melt in your hand (m.p. 29.76 °C).
Germanium #elementphotos, including a Ge laser diode. Last pic is a geranium. Not the same thing. Is it germane to ask a German if a geranium contain germanium?
Arsenic #elementphotos. Very toxic, but has its uses, such as the GaAlAs LED shown in pic 2.
Selenium #elementphotos. Pellets look good enough to eat (very bad idea), while the plates have a beautiful mirror finish. White compound is selenium oxide (SeO2).
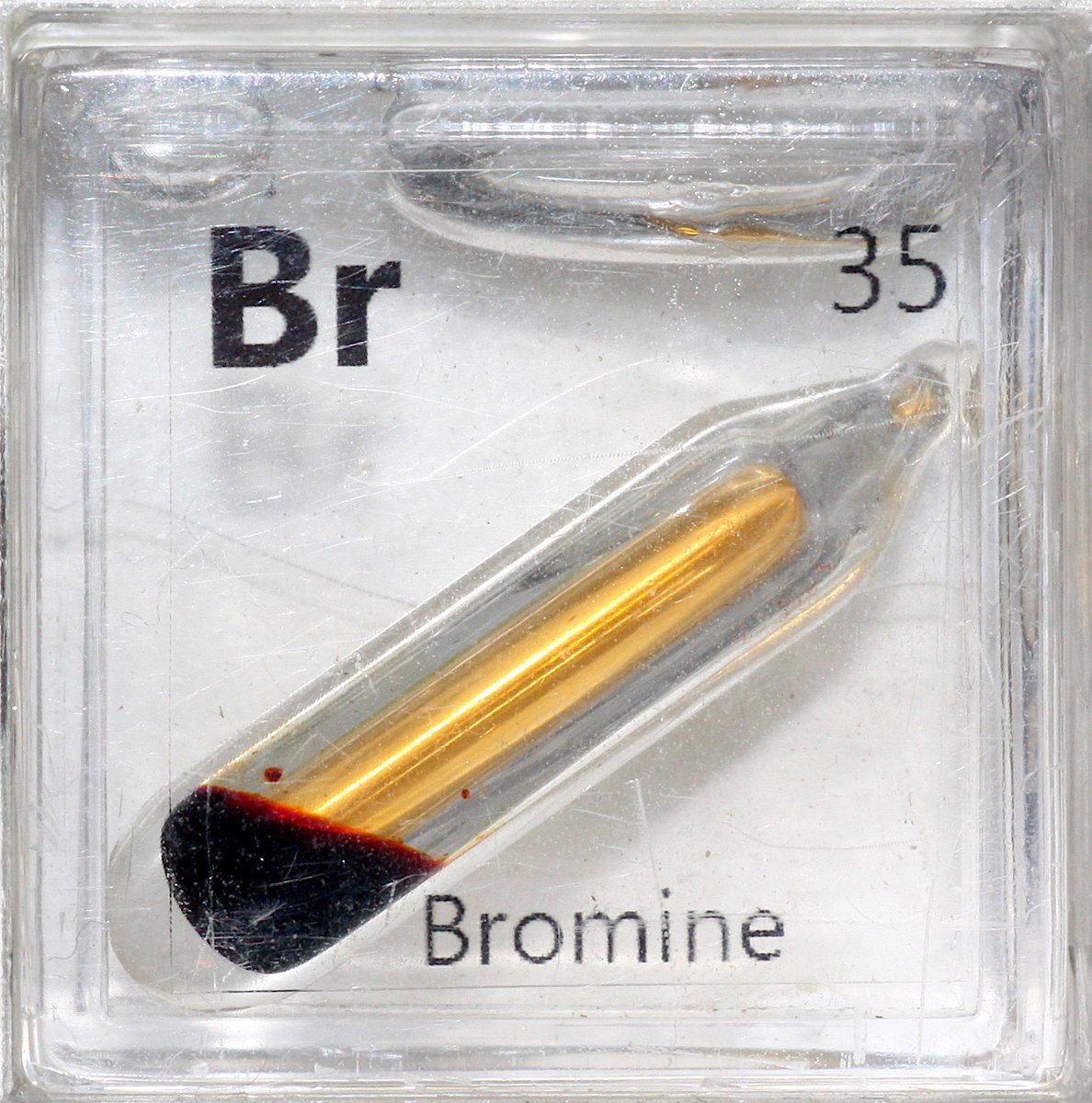
Bromine #elementphotos. White crystals are strontium bromide (SrBr2.6H2O), which has turned out to be quite deliquescent, so I'll need to come up with another compound option.
Krypton #elementphotos. Not the green glowing rocks of Superman, but a colourless gas that glows blue in a discharge tube.
Rubidium #elementphotos. True colour is silver-grey, but seems to take on a bronze colour in the smaller ampoules, perhaps in the sealing process. Rb melting point is just 39 °C. White compound is rubidium chloride (RbCl).
Strontium #elementphotos. Pale blue crystal is celestite, SrSO4.
Yttrium #elementphotos. White powder is yttrium oxide (Y2O3).
Zirconium #elementphotos, including some sparkly cubic zirconia (ZrO2) crystals. The cubic phase actually requires dopants (Ca or Y) to be stable at room temperature; undoped, zirconia is normally monoclinic at RT. There's also a tetragonal phase.
Niobium #elementphotos. Anodising of the grey metal produces oxide coatings that vary in colour depending on the depth of the oxide layer.
Molybdenum #elementphotos. Light grey crystal is Molybdenite, MoS2.
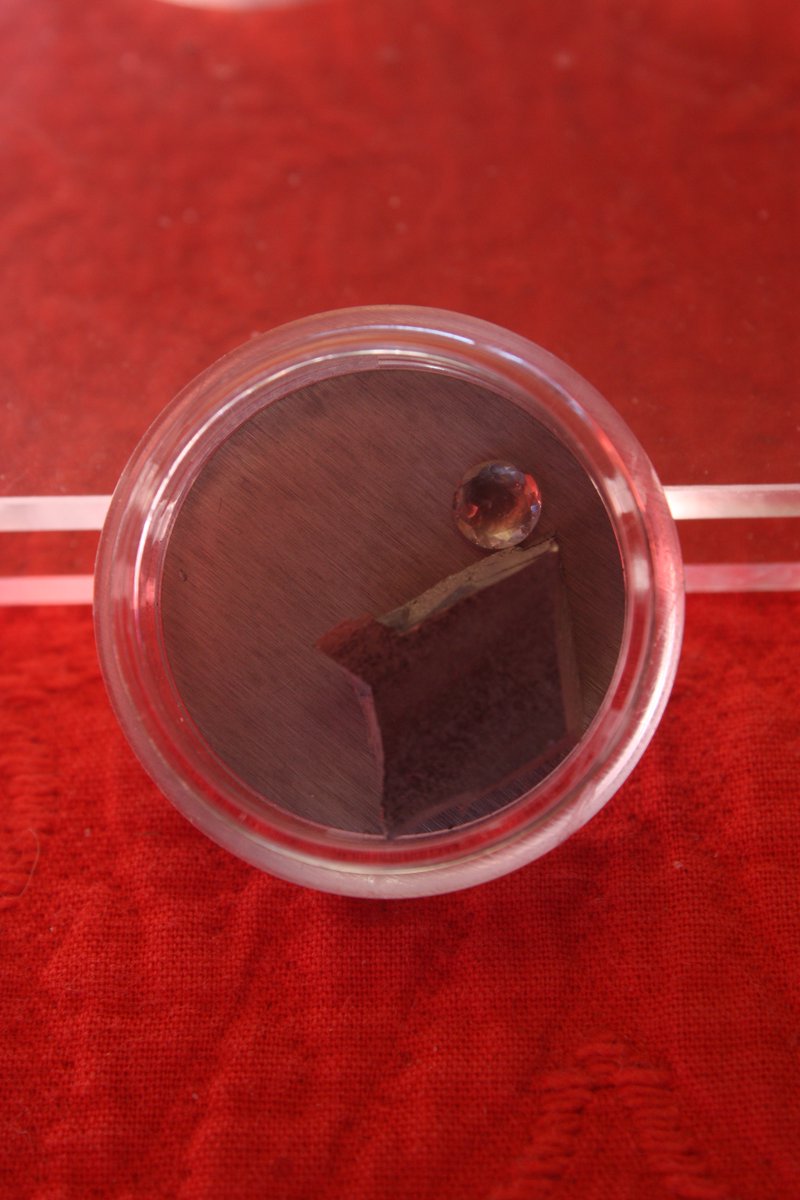
Technetium #elementphotos. All Tc is radioactive and difficult to obtain - these ampoules contain solutions of the radiopharmaceuctical sodium pertechnetate (Na[TcO4]). Starts as Mo-99, which decays to Tc-99m (half-life 6hrs), then to more stable Tc-99 (t1/2 = 211K yrs).
Ruthenium #elementphotos. Red powder is ruthenium acetylacetonate (Ru(acac)3).
Rhodium #elementphotos. Dark compound is rhodium chloride (RhCl3.xH2O). Currently the most expensive non-radioactive compound. Donations welcome.
Palladium #elementphotos. Red compound is potassium palladium chloride (K2PdCl4). The value of Pd has soared past the traditionally more expensive Pt in recent years due to its use in catalytic converters.
Silver #elementphotos.
Cadmium #elementphotos. White compound is cadmium sulfate (CdSO4.2.67H2O).
Indium #elementphotos. Ampoule contains some home-made galinstan, an alloy of gallium, indium and tin which is a liquid at room temperature.
And since we're up to indium in the #elementphotos series, here's an earlier tweet showing just how soft indium really is. https://twitter.com/SBattenResearch/status/1138452872096636929
Tin #elementphotos. White compound is tin chloride (SnCl2.2H2O).
Antimony #elementphotos. True to its metalloid nature it's both soft and brittle. White compound is triphenylantimony dichloride {(C6H5)3SbCl2}.
Tellurium #elementphotos. A silver-indium-antimony-tellurium (AgInSbTe) is used in the reflective layer of CD-RW discs.
Iodine #elementphotos. White crystalline powder is potassium iodide (KI). From black to white, all because of an electron!
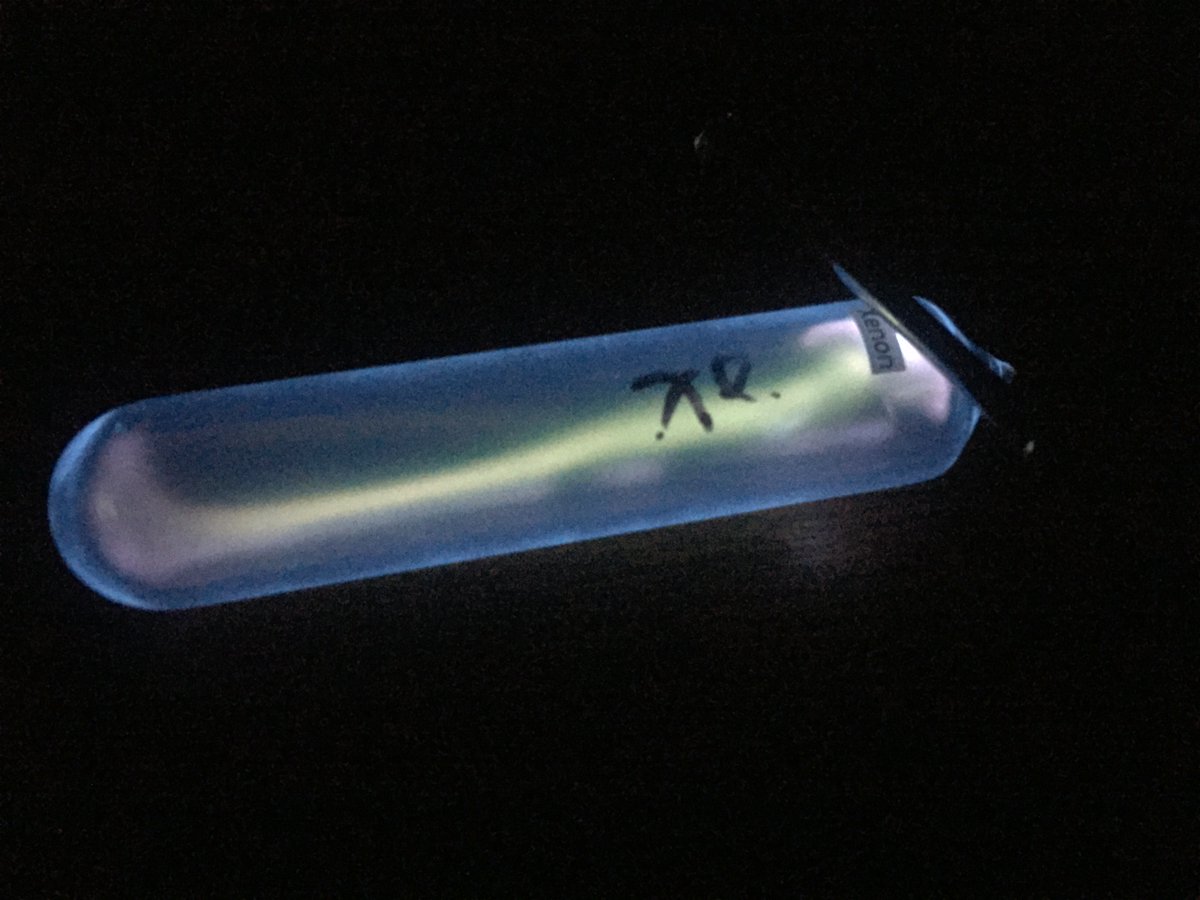
Xenon #elementphotos.
And since we're up to xenon in the #elementphotos, I'll link to this old tweet again. https://twitter.com/SBattenResearch/status/1194118143976693762
Caesium #elementphotos. Cs is *very* reactive but has a lovely golden colour and a melting point of only 28.5 °C, meaning that on a hot summer day it joins mercury and bromine as a liquid element. White powder is caesium iodide (CsI).
Barium #elementphotos. White compound is barium carbonate (BaCO3), while the black pellet is a 123 superconductor (YBa2Cu3O7-x) with a critical temperature of 95 K.
Followup on the barium #elementphotos: here's the superconducting pellet in action! https://twitter.com/SBattenResearch/status/1295338635349303297
Another view of the barium-containing superconducting pellet in action, from our undergraduate labs. Magnet is levitating above the superconductor due to the Meissner effect. #elementphotos
Lanthanum #elementphotos. Like all the early lanthanoids, it oxidises easily. Though stored here in ampoules under inert atmospheres, oxidation is still apparent on at least one of the samples. White compound is lanthanum nitrate (La(NO3)3.6H2O).
Cerium #elementphotos. Beautiful orange compound is ceric ammonium nitrate ((NH4)2Ce(NO3)6).
Praseodymium #elementphotos. Black compound is praseodymium oxide (Pr6O11).
Neodymium #elementphotos. Metal disc is a "neodymium magnet" (actually Nd2Fe14B), while the pink crystals are neodymium nitrate (Nd(NO3)3.6H2O).
Promethium #elementphotos. Promethium is radioactive, with about the only feasible source being old watches that use Pm-based paints for glow-in-the-dark displays. I managed to find & cannibalise one - hands went in one element set, 'dots' on dial went into others.
Samarium #elementphotos. Sm is sensitive to oxidation, as can be seen for these samples. Metal pellet is a samarium-cobalt (SmCo5) magnet.
Europium #elementphotos. White compound is europium oxide (Eu2O3), widely used in phosphors in TV sets. UV light induces a bright fluorescence, as seen here.
Gadolinium #elementphotos. Colourless crystals are gadolinium chloride (GdCl3.xH2O).
Terbium #elementphotos. Black compound is terbium oxide (Tb4O7).
Dysprosium #elementphotos. White compound is dysprosium triflate (Dy(O3SCF3)3). Love the texture on some of these samples.
Holmium #elementphotos. Pink compound is holmium oxide (Ho2O3).
Erbium #elementphotos. Pink compound is erbium oxide (Er2O3).
Thulium #elementphotos. White compound is thulium oxide (Tm2O3).
Ytterbium #elementphotos. Elemental ytterbium is surprisingly soft (similar to zinc), has a lovely golden sheen, and I detected a faint 'burning' smell (likely due to slow oxidation) when cutting some into pieces. White compound is ytterbium oxide (Yb2O3).
Lutetium #elementphotos. White compound is lutetium oxide (Lu2O3).
Hafnium #elementphotos. White compound is hafnium oxychloride (HfOCl2.8H2O).
Tantalum #elementphotos, with a pair of tantalum capacitors thrown in for good measure.
Tungsten #elementphotos. White compound is tungsten hexacarbonyl (W(CO)6).
Rhenium #elementphotos. White powder is rhenium pentacarbonyl chloride (Re(CO)5Cl).
Osmium #elementphotos. Osmium metal has a distinctive grey-blue sheen, and is the densest stable element. Pen nib is an alloy of osmium and iridium known as 'osmiridium'.
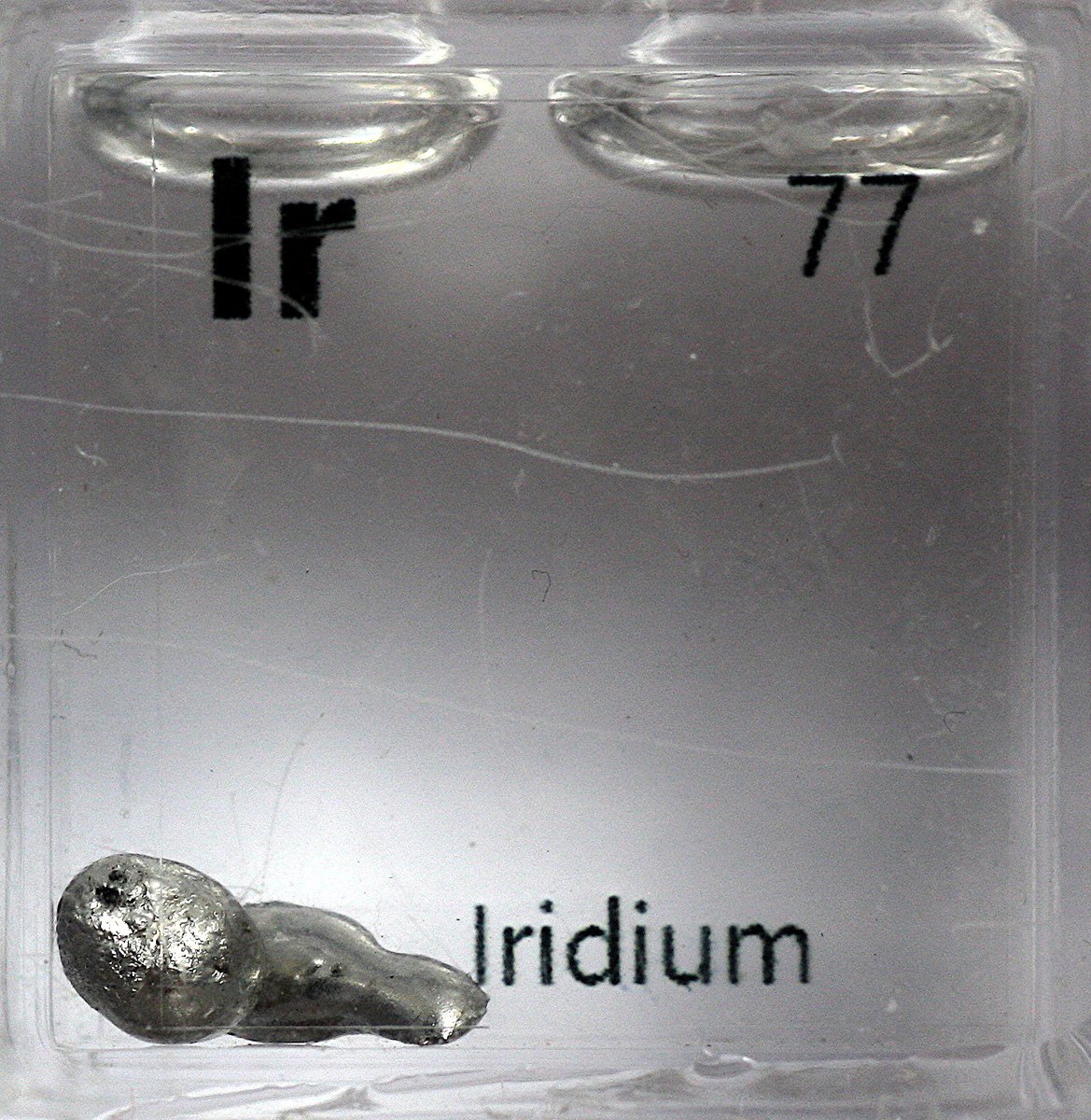
Iridium #elementphotos. Still trying to source a compound/mineral/gadget/doohicky with Ir in it; would love to get a sample of the K-T boundary.
Platinum #elementphotos. Yellow compound is (1,5-cyclooctadiene)diiodoplatinum(II) (C8H8)PtI2.
Gold #elementphotos, including genuine Australian gold nuggets, and gold leaf.
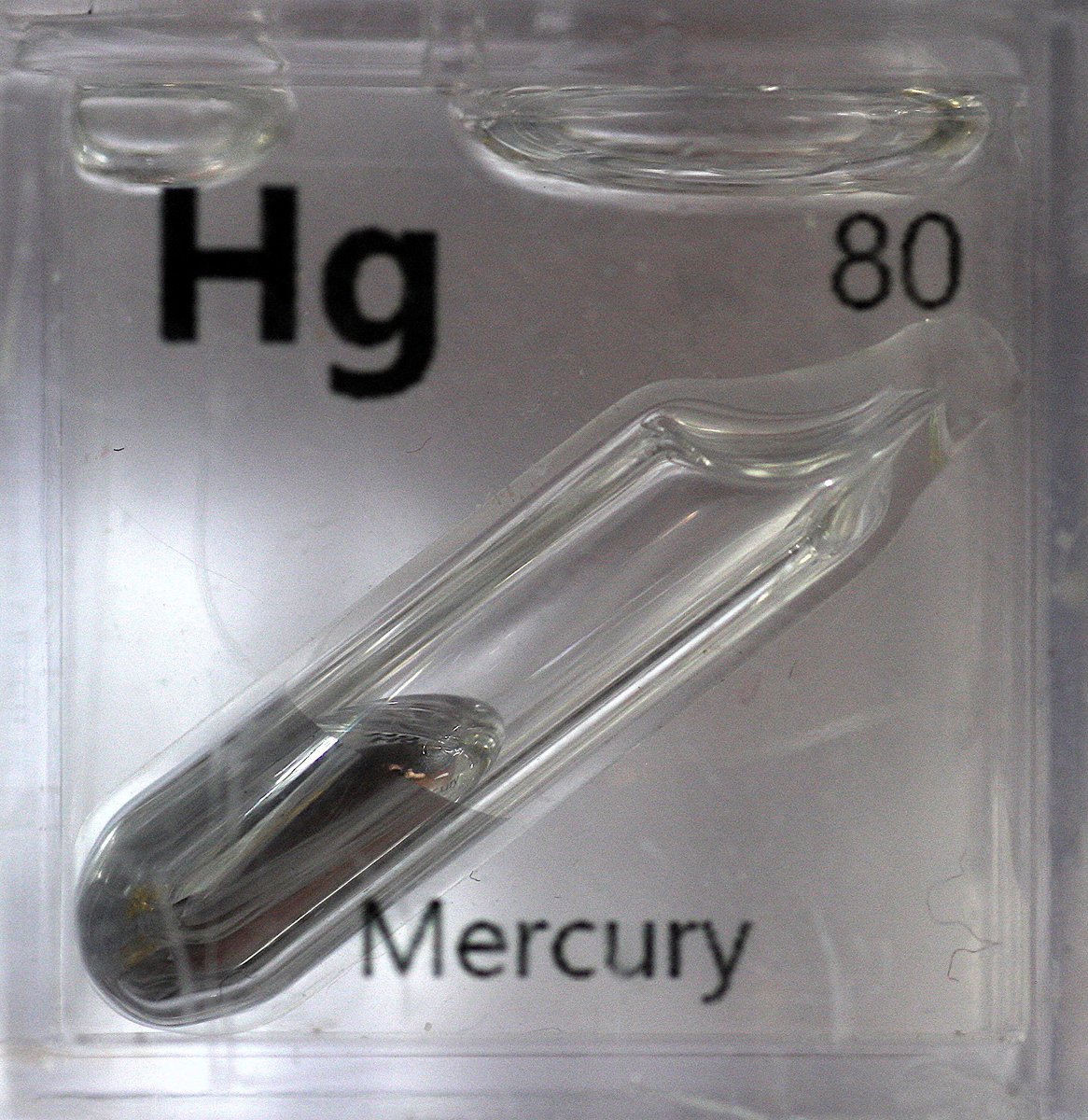
Mercury #elementphotos. The happy little chap is made of cinnabar (HgS).
Thallium #elementphotos. White compound is thallium acetate (Tl(O2CCH3)).
Lead #elementphotos. White compound is lead acetate (Pb(O2CCH3)2.3H2O).
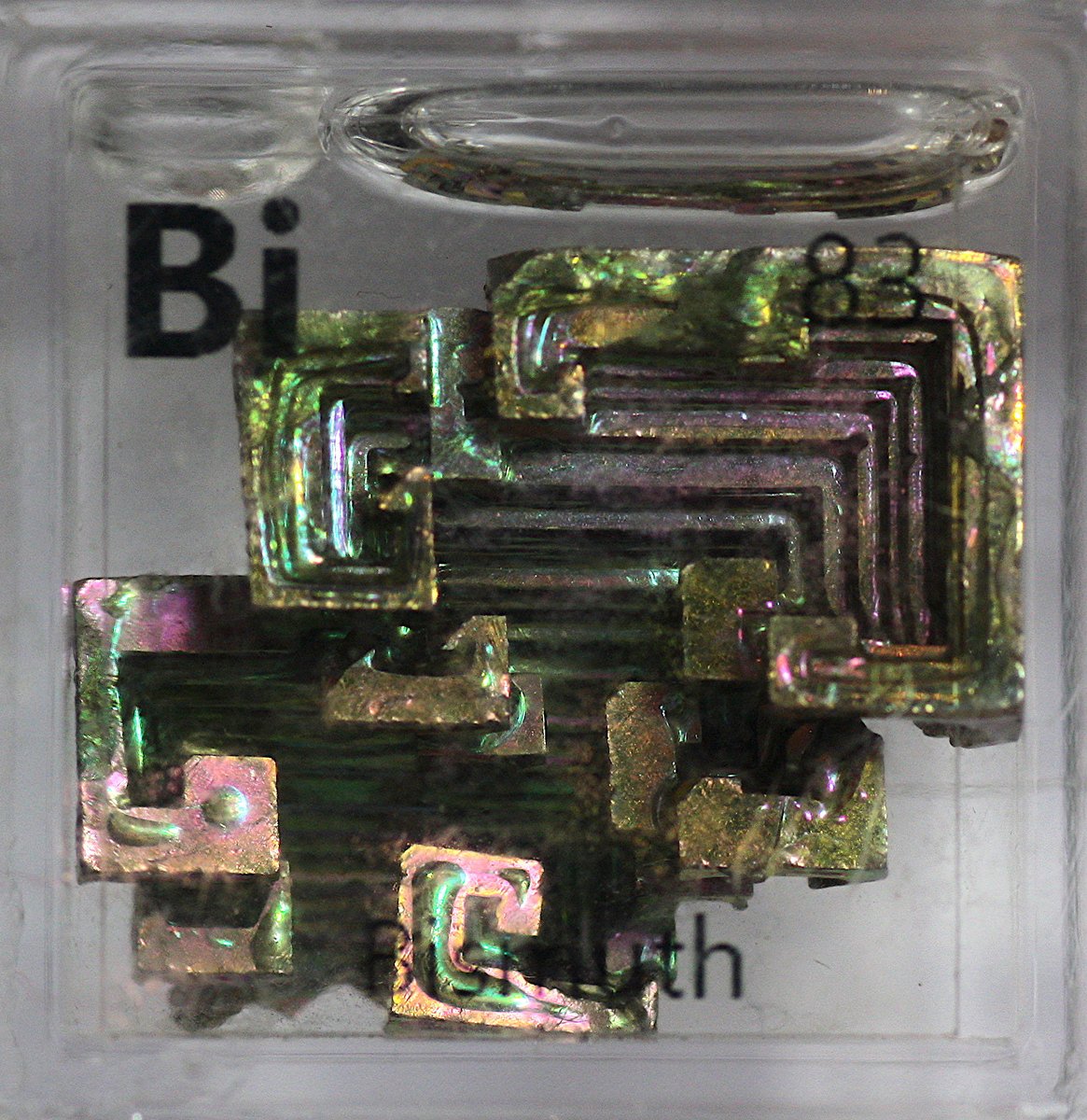
Bismuth #elementphotos. Bi naturally has a beautiful silver look, but when crystallised in the air it forms an oxide layer on the surface. Different depths of oxide layer give different colours (same effect happens in an oil slick), producing lovely multicoloured crystals.
More #elementphotos of my larger bismuth crystals.
Polonium #elementphotos. All isotopes of Po are radioactive, and the only source of Po I could find were "Staticmaster" brushes, like the one shown below. These contain Po-210 strips emit alpha particles which ionise the air and help remove static on e.g. vinyl records. 1/2
So I pulled out the Po-coated metal strips and used these as my 'samples'. However, given Po-210 has a half-life of 138 days, and these strips are from old brushes manufactured in the 1980s, there's likely no Po atoms left! 2/2
Astatine #elementphotos. At is highly radioactive with a very short half-life, which provides a very big challenge. So I've represented it with a piece of uranium ore as it is a (fleeting) member of a U isotope decay chain. Similar approaches have been used for Fr, Ac, Pa.
Radon #elementphotos. Rn is a radioactive gas, so my 'samples' rely on the decomposition of other radioactive elements, in this case radium (watch hand) or uranium (ore sealed in an ampoule to contain the tiny amount of Rn gas as it's produced).
Radium #elementphotos. Radioactive Ra salts are used (rarely nowadays) in luminescent paints for things like watch hands and dials.
Thorium #elementphotos. The rods in the vial are 2%Th / 98% W (used as electrodes in welding), while all the other samples are pure Th (OK, plus some impurities due to the radioactive decay of Th...).
Uranium #elementphotos. Yellow powder is uranyl acetate (UO2(O2CCH3)2.2H2O). Green glass contains uranium salts doped into the glass to make it fluoresce in UV light.
Neptunium and Americium #elementphotos. At the heart of an ionisation-type smoke detector is a little button that contains a small amount of americium dioxide (AmO2). The Am241 (halflife 432 yrs) decays to Np-237 (halflife 2M yrs), and thus the Am is slowly being replace by Np.
Plutonium #elementphotos. Uranium ore (brown rock) naturally contains trace amounts of Pu (neutrons from decaying U-238 can strike other U-238 atoms to give Pu). Green glass-like rocks are Trinitite, which is a residue from the 1945 Pu-based Trinity nuclear bomb test.
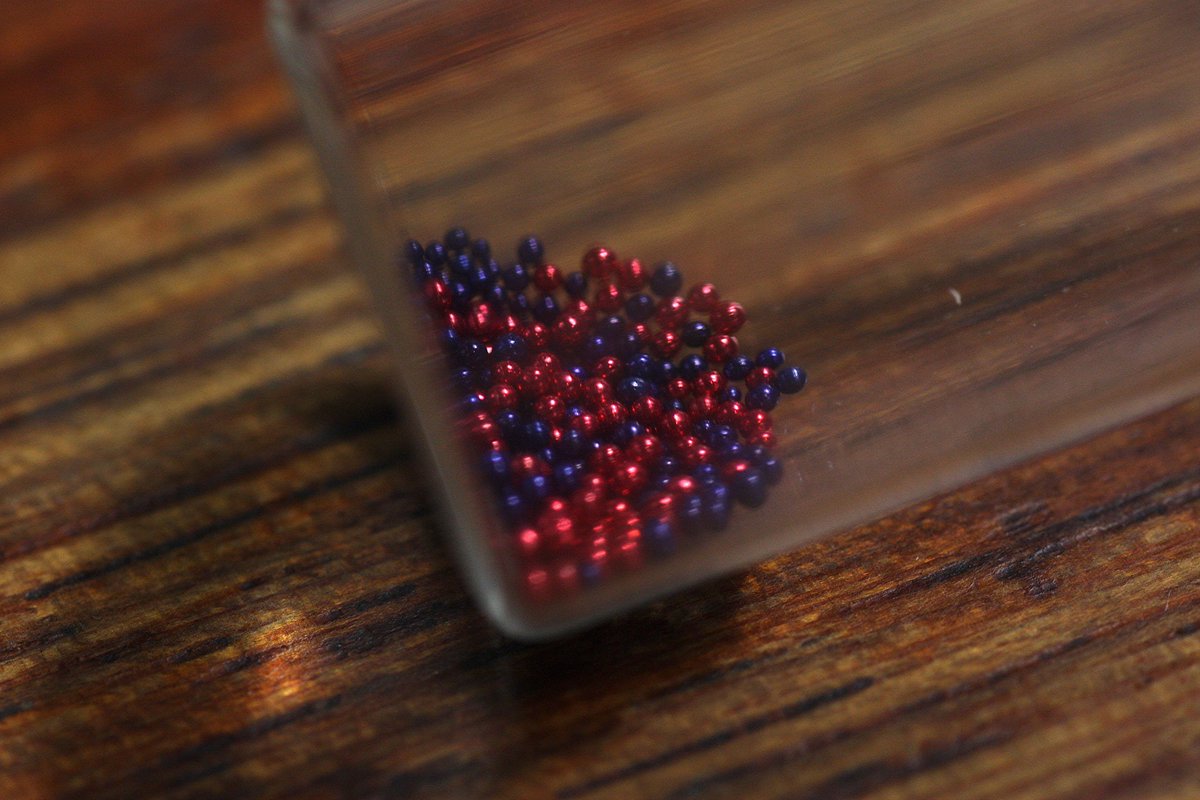
Last of the #elementphotos - Curium and beyond. The elements from here are all man-made and mostly too radioactive & short-lived to collect. One option I'm looking at is to represent the nucleus of one isotope of each with little beads - red for protons, blue for neutrons.
And with that, the #elementphotos thread is done. Phew! I hope the two men and a dog still following along enjoyed it. What started as a lockdown project for my own amusement lasted well past lockdown, but once started I was determined to get to the end!

 Read on Twitter
Read on Twitter

























































































































![Technetium #elementphotos. All Tc is radioactive and difficult to obtain - these ampoules contain solutions of the radiopharmaceuctical sodium pertechnetate (Na[TcO4]). Starts as Mo-99, which decays to Tc-99m (half-life 6hrs), then to more stable Tc-99 (t1/2 = 211K yrs). Technetium #elementphotos. All Tc is radioactive and difficult to obtain - these ampoules contain solutions of the radiopharmaceuctical sodium pertechnetate (Na[TcO4]). Starts as Mo-99, which decays to Tc-99m (half-life 6hrs), then to more stable Tc-99 (t1/2 = 211K yrs).](https://pbs.twimg.com/media/EjEzMtBUcAINXsM.jpg)
![Technetium #elementphotos. All Tc is radioactive and difficult to obtain - these ampoules contain solutions of the radiopharmaceuctical sodium pertechnetate (Na[TcO4]). Starts as Mo-99, which decays to Tc-99m (half-life 6hrs), then to more stable Tc-99 (t1/2 = 211K yrs). Technetium #elementphotos. All Tc is radioactive and difficult to obtain - these ampoules contain solutions of the radiopharmaceuctical sodium pertechnetate (Na[TcO4]). Starts as Mo-99, which decays to Tc-99m (half-life 6hrs), then to more stable Tc-99 (t1/2 = 211K yrs).](https://pbs.twimg.com/media/EjEzQdbU4AA5gaB.jpg)