Thread 1/15
Can you explain what #QuantumMechanics is to a 13-,14- year-old boy?
A really arduous undertaking, I would say almost a challenge.
I tried to do it through an imaginary dialogue with Marco, an equally imaginary 13-,14-year-old boy.
My article https://bit.ly/3fn51hx
https://bit.ly/3fn51hx
Can you explain what #QuantumMechanics is to a 13-,14- year-old boy?
A really arduous undertaking, I would say almost a challenge.
I tried to do it through an imaginary dialogue with Marco, an equally imaginary 13-,14-year-old boy.
My article
 https://bit.ly/3fn51hx
https://bit.ly/3fn51hx
2/
Marco: Do you explain to me what #QuantumMechanics is, please?
Me: Mechanics...what?
Marco: Q-u-a-n-t-u-m!
Me: But where did you hear about it?
Marco: On Facebook!
Me: On Facebook ???
Marco: Do you explain to me what #QuantumMechanics is, please?
Me: Mechanics...what?
Marco: Q-u-a-n-t-u-m!
Me: But where did you hear about it?
Marco: On Facebook!
Me: On Facebook ???
3/
Marco: That's right.They talked about photons, quanta, Planck, Schroedinger, Dirac, Heisenberg, Einstein, & other things I didn't understand at all.
Me: Of course! They are difficult things for adults, let alone for a 13- year- old boy, almost 14, although curious, like you!!!
Marco: That's right.They talked about photons, quanta, Planck, Schroedinger, Dirac, Heisenberg, Einstein, & other things I didn't understand at all.
Me: Of course! They are difficult things for adults, let alone for a 13- year- old boy, almost 14, although curious, like you!!!
4/
Marco: Please, I'm all ears!
Me: Okay I try, but I don't assure you of the result, that is, that you will understand or better to say that I will make you understand ...
Let's start with the term "Mechanics" which simply means "Physics". There is Physics and Physics!
Marco: Please, I'm all ears!
Me: Okay I try, but I don't assure you of the result, that is, that you will understand or better to say that I will make you understand ...
Let's start with the term "Mechanics" which simply means "Physics". There is Physics and Physics!
5/
For example Classical and Quantum, the areas that interest us in particular.
Classical Physics is linked to Isaac Newton: it is, in fact, called Newtonian Mechanics.
Do you remember? We talked about it when we treated the principles of classical dynamics, in fact.
For example Classical and Quantum, the areas that interest us in particular.
Classical Physics is linked to Isaac Newton: it is, in fact, called Newtonian Mechanics.
Do you remember? We talked about it when we treated the principles of classical dynamics, in fact.
6/
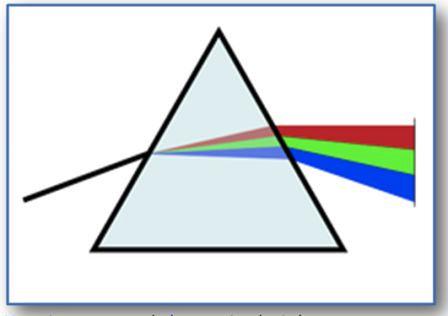
Marco: Yes, I do! Law of inertia, F = m x a, Law of action and reaction ... And then the Newton's prism and the dispersion of light, and also the diffraction of light that we observed on the CD.
Me:Bravo! I see you have a good memory. So you also remember the visible spectrum
Marco: Yes, I do! Law of inertia, F = m x a, Law of action and reaction ... And then the Newton's prism and the dispersion of light, and also the diffraction of light that we observed on the CD.
Me:Bravo! I see you have a good memory. So you also remember the visible spectrum
7/
of light, i.e. the electromagnetic spectrum (btwn red & violet), including all the colors perceivable by the human eye.
Well, between the end of the 19th century and the beginning of the 20th one, some experimental results questioned the completeness of Newtonian mechanics.
of light, i.e. the electromagnetic spectrum (btwn red & violet), including all the colors perceivable by the human eye.
Well, between the end of the 19th century and the beginning of the 20th one, some experimental results questioned the completeness of Newtonian mechanics.
8/
In particular, the set of spectral lines, obtained by analyzing the light emitted by incandescent gases or by gases subjected to electric discharge, was at odds with the atomic model of Ernest Rutherford.
We studied this too, do you remember?
In particular, the set of spectral lines, obtained by analyzing the light emitted by incandescent gases or by gases subjected to electric discharge, was at odds with the atomic model of Ernest Rutherford.
We studied this too, do you remember?
9/
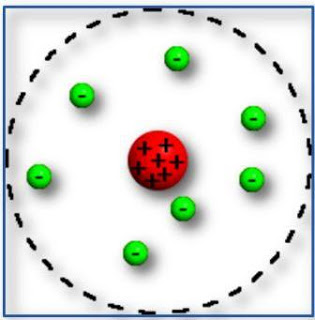
Marco: Of course! Rutherford atomic model, where the atomic nucleus and the electrons that rotate around it are thought to be present, like the planets of the solar system around the sun. Isn't it?
Me: That's right! Furthermore, the study of the black- body spectrum and the
Marco: Of course! Rutherford atomic model, where the atomic nucleus and the electrons that rotate around it are thought to be present, like the planets of the solar system around the sun. Isn't it?
Me: That's right! Furthermore, the study of the black- body spectrum and the
10/
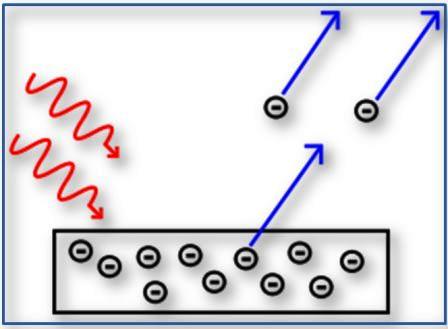
photoelectric effect suggested that electromagnetic radiation had a dual behavior (undulatory and corpuscular) during the interaction processes with matter.
Marco: Easy to you! What are the black-body spectrum and the #photoelectriceffect?
photoelectric effect suggested that electromagnetic radiation had a dual behavior (undulatory and corpuscular) during the interaction processes with matter.
Marco: Easy to you! What are the black-body spectrum and the #photoelectriceffect?
11/
Me: The black- body in physics is a kind of ideal radiator, which absorbs all the electromagnetic radiation that hits it and, therefore, neither reflects nor transmits any energy, so appearing black, according to the classic interpretation of the bodies' color.
Me: The black- body in physics is a kind of ideal radiator, which absorbs all the electromagnetic radiation that hits it and, therefore, neither reflects nor transmits any energy, so appearing black, according to the classic interpretation of the bodies' color.
12/
The black- body therefore absorbs all the incident energy and, for the conservation of energy, re-radiates all the amount of absorbed energy.
The light emitted by a black- body is called black- body radiation and the energy density radiated black- body spectrum. All clear?
The black- body therefore absorbs all the incident energy and, for the conservation of energy, re-radiates all the amount of absorbed energy.
The light emitted by a black- body is called black- body radiation and the energy density radiated black- body spectrum. All clear?
13/
Marco: Not sure! You spoke of an ideal black- body, so in reality such an object does not exist, if I have understood correctly.
Me: You understood very well! I would add that the difference between the spectrum of an object and that one of an ideal black-body
Marco: Not sure! You spoke of an ideal black- body, so in reality such an object does not exist, if I have understood correctly.
Me: You understood very well! I would add that the difference between the spectrum of an object and that one of an ideal black-body
14/
makes it possible to identify the chemical composition of that object and therefore to identify it.
Sounds interesting to me, doesn't it?
A real joy for chemists :).
makes it possible to identify the chemical composition of that object and therefore to identify it.
Sounds interesting to me, doesn't it?
A real joy for chemists :).
15/
Now, if you're curious, may continue to read my article using the automatic translator at this link:
 https://bit.ly/3fn51hx
https://bit.ly/3fn51hx
#quantumphysics
Now, if you're curious, may continue to read my article using the automatic translator at this link:
 https://bit.ly/3fn51hx
https://bit.ly/3fn51hx #quantumphysics

 Read on Twitter
Read on Twitter