EU accused of risking lives in standoff over coronavirus medicines produced in Britain after Brexit . Story plus thread. 1/ https://www.telegraph.co.uk/politics/2020/07/11/eu-accused-risking-lives-standoff-coronavirus-medicines-produced/
Brussels has rejected UK proposals to remove barriers to trade in vaccines, medicines & medical devices in the Brexit trade negotiations. 2/
This means, as things stand, medicines, devices & any UK produced coronavirus vaccine will need to be tested twice in the newly separated UK & EU jurisdictions, which could lead to delays in supplies and cost UK & EU companies hundreds of millions of pounds every year.
3/
3/
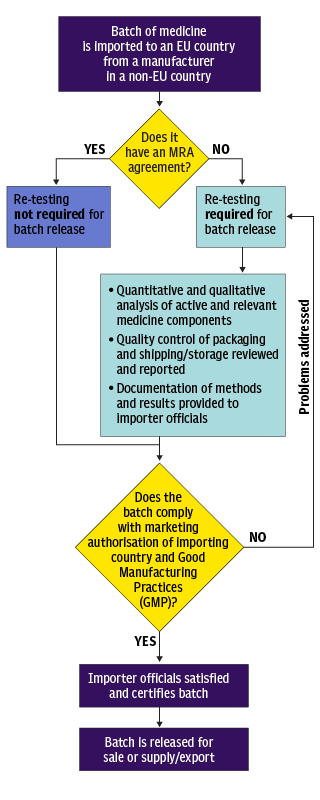
Delays for medicines are estimated as up to 6 weeks on average but substantially longer, up to 4 months, for complicated products such as vaccines. Here’s a chart showing how batch testing works. Each batch, which can be v large or as small as 1 needs to be tested. 4
EU diplomats and sources in Brussels insist they are not worried. Approx 7,500 certifications were transferred to EU ahead of last no deal cliff edge. Oe source says pharma industry has had plenty of time to prepare for end of Brexit transition. 5.
BUT UK is no 1 supplier of medicines to the EU by volume and the third in terms of value. UK-EU trade was worth €32bn in 2018 with Britain buying €20bn worth of medicines from the EU and selling €12 bn into the bloc.
6
6
UK supplies account for a third of EU demand and UK manufacturers import 54 percent of their ingredients from the bloc. 7/
Trade expert @SamuelMarcLowe says "UK will compete w/ China to be 3rd-biggest supplier of medical products to EU even if new trade frictions between the EU and UK reduce trade. It's in the EU’s interest to engage with the UK’s request." His analysis: 8 https://www.cer.eu/publications/archive/bulletin-article/2020/securing-europes-medical-supply-chains-against-future8/
Coronavirus exposed EU dependence on certain suppliers like China for medical imports. Onshoring testing from UK to EU shrinks rather than diversifies the pool of suppliers, increasing EU’s dependence. Brexit politics trumping pragmatism & public health? 9/
UK wants mutual recognition of Good Manufacturing Practice site inspections and batch testing certificates to prevent multiple inspections and testing of medicines. It wants “mutual recognition agreements for conformity assessments” for medical devices. 10/
UK wants to stay in Official Control Batch Release Network., which is a group of specialist labs testing vaccines. It includes UK’s National Institute for Biological Standards and Control (NIBSC), which was responsible for the scaling up of vaccines for Ebola & Meningitis C.
11
11
If London or Oxbridge come up with a coronavirus vaccine next year, it will have to be tested twice and shipped under strict storage conditions. And if the results don't match - delays. 12/
But isn’t this (yawn) cherry-picking? Well the EU already has mutual recognition for batch testing of medicines with the USA, Switzerland, Japan, Canada, Israel, Australia and New Zealand on public health grounds. 13/
Israeli labs are part of the Official Control Batch Release Network.*
*it is all well precedented in other trade deals (yawn)
14/
*it is all well precedented in other trade deals (yawn)
14/
Pharma wrote to Barnier “Failing to agree key medicine provisions [...] will only introduce unnecessary uncertainty and disruption & divert resources at a time when governments and industry need to fully focus on finding a solution to end this pandemic 15/ https://www.efpia.eu/news-events/the-efpia-view/statements-press-releases/need-for-a-mutual-recognition-agreement-between-the-eu-and-uk/?utm_source=POLITICO.EU&utm_campaign=2de3989e83-EMAIL_CAMPAIGN_2020_06_18_11_11&utm_medium=email&utm_term=0_10959edeb5-2de3989e83-190429029
EU doesn't look like budging yet. Barnier’s speeches suggest he will hold firm. 1 EU diplomat told me caving on this would breach his mandate. Debatable. 16/ https://www.telegraph.co.uk/news/2020/06/10/london-should-not-european-financial-hub-brexit-says-michel/
"During its 47 years of membership, the UK built up a strong position in the EU market in a number of strategic areas, financial, services, businesses, and legal services, and also regulation and certification," Barnier said in 1 speech
17/
17/
"Do we really want to consolidate the UK position as a certification hub for the EU, knowing that it already controls some 15 to 20 percent of the EU certification market?" Mr Barnier said.
18/
18/
On Thursday, the commission published a readiness notice for the end of transition on Dec 31.
“Testing and batch release sites will need to be located in the European Union,”it read. 19/
https://ec.europa.eu/info/sites/info/files/brexit_files/info_site/com_2020_324_2_communication_from_commission_to_inst_en_0.pdf
“Testing and batch release sites will need to be located in the European Union,”it read. 19/
https://ec.europa.eu/info/sites/info/files/brexit_files/info_site/com_2020_324_2_communication_from_commission_to_inst_en_0.pdf
UK Government official said: “The UK's proposals on medicines and medical devices would provide significant benefits to patients, industry and regulators in the EU and UK, including by avoiding any new delays to access to medicines.
20/
20/
“We hope that the EU will engage with the proposal so that we can deliver these mutual benefits, which are particularly important in the current public health context.”
21
21
Now UK hasnt covered itself in glory on securing coronavirus supplies either..22/ https://www.telegraph.co.uk/politics/2020/03/26/boris-johnson-criticised-ignoring-eu-coronavirus-ventilators/
But this isnt about whether Brexit is right or wrong, it is about barriers to trade in medicines at time of global health crisis. And what are trade negotiations for? To remove barriers.*
*Yes Brexit creates trade barriers.
23/
*Yes Brexit creates trade barriers.
23/
Mutual regulatory recognition is EU’s choice. But it is odd to offer this to the US on public health grounds, which the EU doesn’t have a trade deal with, and not the UK.
If stance holds - feels like a political rather than pragmatic choice. & a misjudgment
24.
If stance holds - feels like a political rather than pragmatic choice. & a misjudgment
24.
There is more detail in the story below, which will be published in tomorrow's Sunday Telegraph ( #buyapaper).
If you don't have a subscription, you can register to get access to the full story. ENDS https://www.telegraph.co.uk/politics/2020/07/11/eu-accused-risking-lives-standoff-coronavirus-medicines-produced/
If you don't have a subscription, you can register to get access to the full story. ENDS https://www.telegraph.co.uk/politics/2020/07/11/eu-accused-risking-lives-standoff-coronavirus-medicines-produced/

 Read on Twitter
Read on Twitter