Our latest #COVID19 preprint “Clinical Sensitivity and Interpretation of PCR and Serological COVID-19 Diagnostics for Patients Presenting to the Hospital”
A thread on the interpretation framework we @MGHpathology are using to integrate PCR & serology testing to diagnose COVID19
A thread on the interpretation framework we @MGHpathology are using to integrate PCR & serology testing to diagnose COVID19
Testing is central to diagnosing SARS-CoV-2 infection, but interpretation for PCR & serology assays can be challenging because the clinical sensitivity changes as the virus clears and the immune response emerges.
Manuscript: https://www.medrxiv.org/content/10.1101/2020.06.19.20135723v1.article-info
Manuscript: https://www.medrxiv.org/content/10.1101/2020.06.19.20135723v1.article-info
Our goal was to examine the clinical sensitivity of two most common COVID-19 diagnostic test modalities, PCR & antibody testing, over the disease course to provide insight into their clinical interpretation in patients presenting to the hospital.
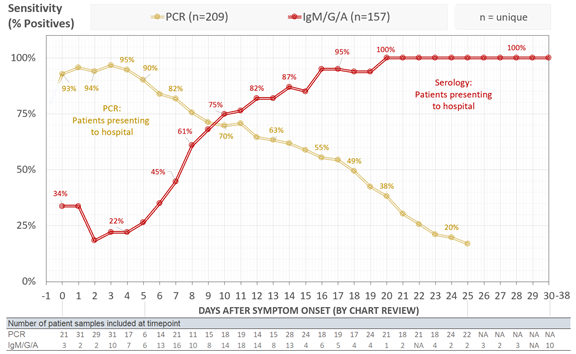
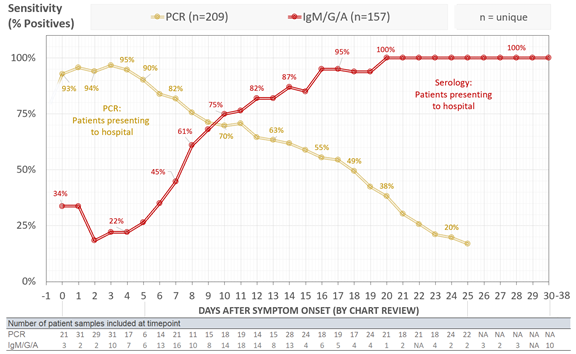
We analyzed all #COVID19 PCR tests conducted by @MGHPathology in the first 2 months of testing. Out of 15k tests on 11k patients, we identified 209 COVID19+ patients with multiple PCR tests that were informative to calculate sensitivity (can't determine false neg from 1 test).
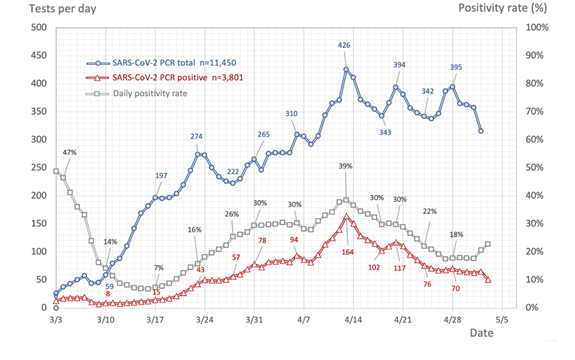
Side note: based on plotting out all of the PCR data, we found the peak of positivity @MassGeneralNews was April 13th. We’ve looked at more recent data and this still holds true.
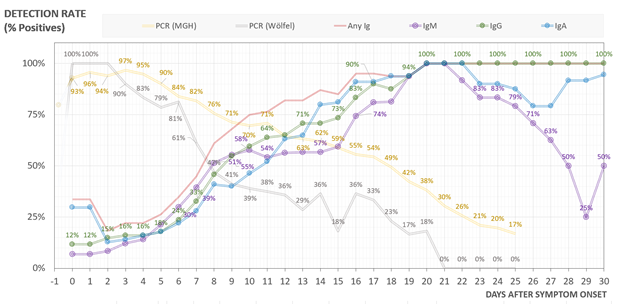
We found that clinical sensitivity of PCR decreased with days post symptom onset with >90% clinical sensitivity during the first 5 days after symptom onset, 70-71% from days 9-11, and 30% at day 21. Median time from symptom onset to admission was 8 days.
See yellow line below:
See yellow line below:
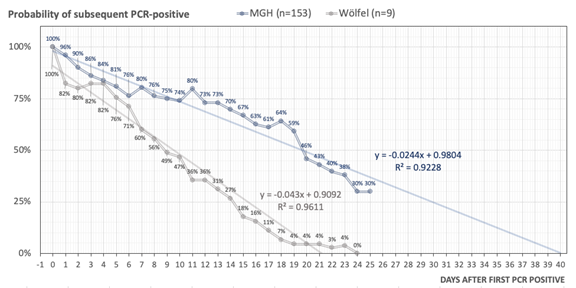
We also modeled how PCR sensitivity decreases over time after the first positive PCR-test (rather than symptom onset). Regression modeling and extension of PCR-positivity decay revealed that in our cohort, NP-swab specimens could stay PCR-positive beyond 20 and up to 40 days
To understand the clinical sensitivity of serology, we used our in-house @MGHPathology and @ragoninstitute ELISA assay that can quantitatively detect IgG, IgM, and IgA. We tested 197 serums from 157 COVID19+ patients.
In contrast with PCR, serological sensitivity increased with days post symptom onset with >50% of patients seropositive by at least one antibody isotype after day 7, >80% after day 12, and 100% by day 21. This is combined detection for any isotype.
See red line below:
See red line below:
Plotted by isotype - seroconversion for COVID *DOES NOT* follow the typical kinetics of IgM followed by IgG/IgA. All appear simultaneously at a cohort level, with IgG or IgA seropositivity preceding IgM responses in some cases. Testing all 3 isotypes increases sensitivity!
With our data above, other published data, and lots of input from pathologists and clinicians, we developed the following 5 diagnostic principles for utilizing and interpreting #COVID19 diagnostic tests:
(1) In symptomatic patients, all interpretations are anchored on days post symptom onset.
Understanding performance characteristics of SARS-CoV-2 diagnostics over the course of the infection is key to interpretation of results. Suggestion: DATE of symptom onset on EMR front page
Understanding performance characteristics of SARS-CoV-2 diagnostics over the course of the infection is key to interpretation of results. Suggestion: DATE of symptom onset on EMR front page
(2) PCR is the diagnostic gold standard during acute infection.
It's the most sensitive and accurate assay to tell if patient is currently infected. While PCR is not perfect, at no point during active infection should serology replace PCR for diagnosis.
It's the most sensitive and accurate assay to tell if patient is currently infected. While PCR is not perfect, at no point during active infection should serology replace PCR for diagnosis.
(3) Clinical sensitivity of PCR decreases with days post symptom onset.
Most patients present to the hospital after symptom onset (median 8 days in our cohort). Disease severity may also influence how long someone stays PCR+. Recognizing both is key to interpreting PCR results
Most patients present to the hospital after symptom onset (median 8 days in our cohort). Disease severity may also influence how long someone stays PCR+. Recognizing both is key to interpreting PCR results
(4) Serological assay sensitivity increases with days post symptom onset.
In our data, serology sensitivity passes PCR sensitivity around day 10.
As we get further out in pandemic, a positive result does not conclusively indicate patient's presenting symptoms are due to COVID19.
In our data, serology sensitivity passes PCR sensitivity around day 10.
As we get further out in pandemic, a positive result does not conclusively indicate patient's presenting symptoms are due to COVID19.
(5) Negative results do not completely preclude SARS-CoV-2 infection.
Ruling out COVID19 is hard. Adding serology to PCR can increase sensitivity. However, there is a window period (day 6-12 from symptom onset in our data) when clinical sensitivity of PCR & serology are <90%.
Ruling out COVID19 is hard. Adding serology to PCR can increase sensitivity. However, there is a window period (day 6-12 from symptom onset in our data) when clinical sensitivity of PCR & serology are <90%.
There are lots of limitations of our study: relatively small n, retrospective design, & selection bias due to the specific setting and testing practice. We evaluated symptomatic, mostly hospitalized patients - cannot derive recommendations for asymptomatic/mildly symptomatic pts
Limitations cont’d: Due to limited availability of tests and time constraints during an ongoing pandemic, we do not have daily samples. Findings for serology are specific to our ELISA assay, which uses RBD antigen (although are similar to other studies)
Limitations cont’d: Finally, our multi-PCR & serology cohorts are largely non-overlapping (n=20/209). To enable additive sensitivity calculations from combined PCR & serology assays, prospective & systematically obtained repeated parallel PCR & serology data is necessary
Even with these limitations, given the ongoing pandemic we think it is important to present this real-world evidence to help clinicians and patients understand the strengths and weaknesses of these assays.
This was a huge group effort that started in March with critical input from our serology task force @MassGeneralNews, @MGHMedicine and @mghpathology. We had multiple MDs chart reviewing each case to get symptom onset date, we had to develop the ELISA and validate 3 PCR assays.
Contributions abound-thanks to my co-authors on twitter: @wilfredo_nk (led ELISA work) @tasillas & @melisanahtar (led PCR work) @MikeGAstudillo @JuliaThierauf @adamsfisch @SchmidtLabHMS @BradEBernstein @advancingcures & all our micro, core, immunology, molecular lab directors!

 Read on Twitter
Read on Twitter