Our lab's first paper ( https://rdcu.be/b49xK ) is now out at @nature. We found that the cytokine IL-18 can drive potent anti-tumor activity from the innate and adaptive immune system, but that its activity is restricted by a high-affinity "decoy receptor" IL-18BP. (1/)
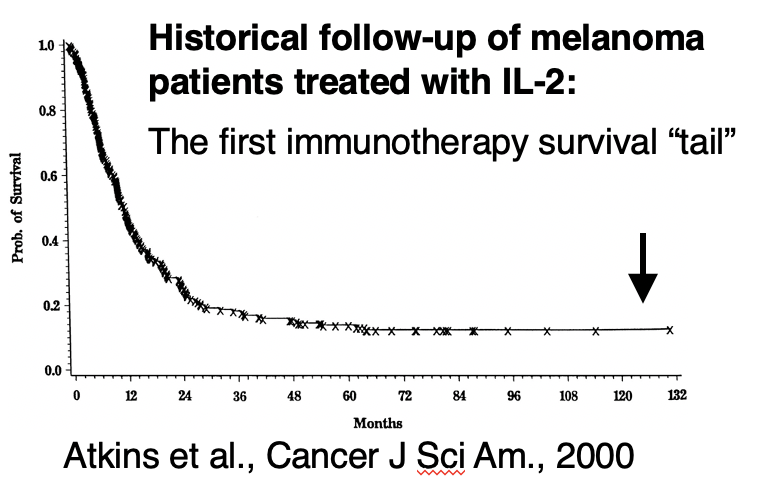
Cytokines were the first modern drugs that unambiguously proved the immune system could be a powerful target against cancer. IL-2 had an ORR of ~15% in metastatic melanoma and renal cell carcinoma, with 6% of patients having complete, durable responses that lasted decades. (2/)
However, cytokines exhibit extensive pleiotropism that limits their therapeutic use. For example, IL-2 activates anti-tumor CD8 & NK cells, but it also stimulates Treg and causes dangerous vascular leak syndrome. Thus, cytokines need to be engineered to be effective drugs (3/).
When we started this project, we wondered if there were cytokines that had been overlooked in cancer immunotherapy. We thus searched for cytokine pathways that were specifically enriched in anti-tumor lymphocytes. This would suggest they could be more specific & effective. (4/)
A sweet paper from @AnaAndersonlab, one of the first scRNAseq papers on tumor infiltrating lymphocytes provided some clues ( https://tinyurl.com/y7gj5agn ). Analyzing their data, we found that IL-18's receptor subunits were enriched in both activated & dysfunctional gene modules. (5/)
We dove into the biology of IL-18. It was very compelling as a target for immunotherapy. The receptor is expressed on the "right" cells (antigen-experienced T cells and NK cells) and it sends a powerful message through MyD88. (6/)
We were interested to learn that IL-18 had been tested in the clinic before, including in a phase 2 trial of over 60 melanoma patients. However, IL-18 bombed. Unlike other cytokines, not due to toxicity, but due to a complete lack of efficacy. This was a shocking paradox... (7/)
This was a largely immunotherapy naive cohort of patients with "hot" tumors: IL-18 should have worked in this setting! Why didn't it? It made us think that perhaps there were inhibitory signals that were present in tumors that were blocking the effect of IL-18. (8/)
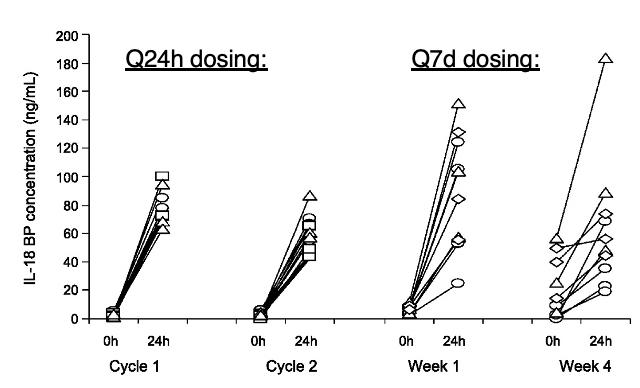
Sure enough, IL-18 is regulated by an ultra-high affinity decoy receptor called IL-18BP. It's part of a feedback loop downstream of IFN-g (and also IL-27 @KingOfPathogens). Predictably, administering IL-18 increases the circulating levels of IL-18BP by 10-100 fold. (9/)
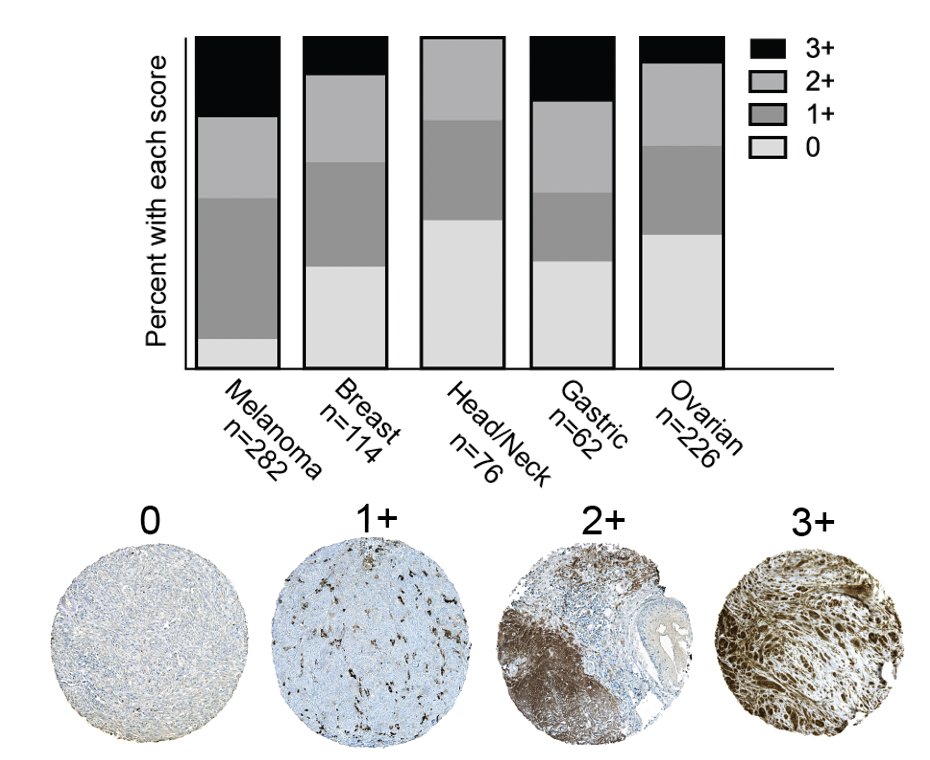
We looked within tumors ourselves using IHC and found that most tumors had at least punctate levels of IL-18BP expression (on macrophages), with many tumors showing extensive staining within tumor and stromal cells. (10/)
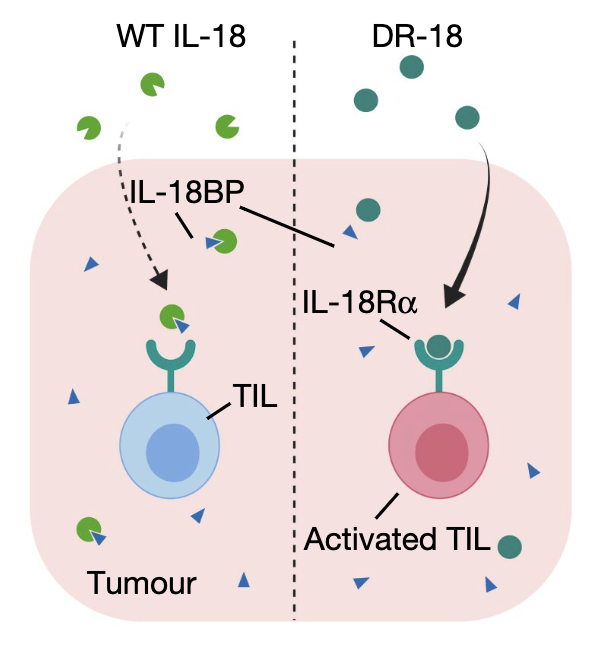
This led us to a very straight-forward hypothesis: Perhaps IL-18 would have been a great drug but for the pesky IL-18BP decoy that was jamming its activity within tumors.
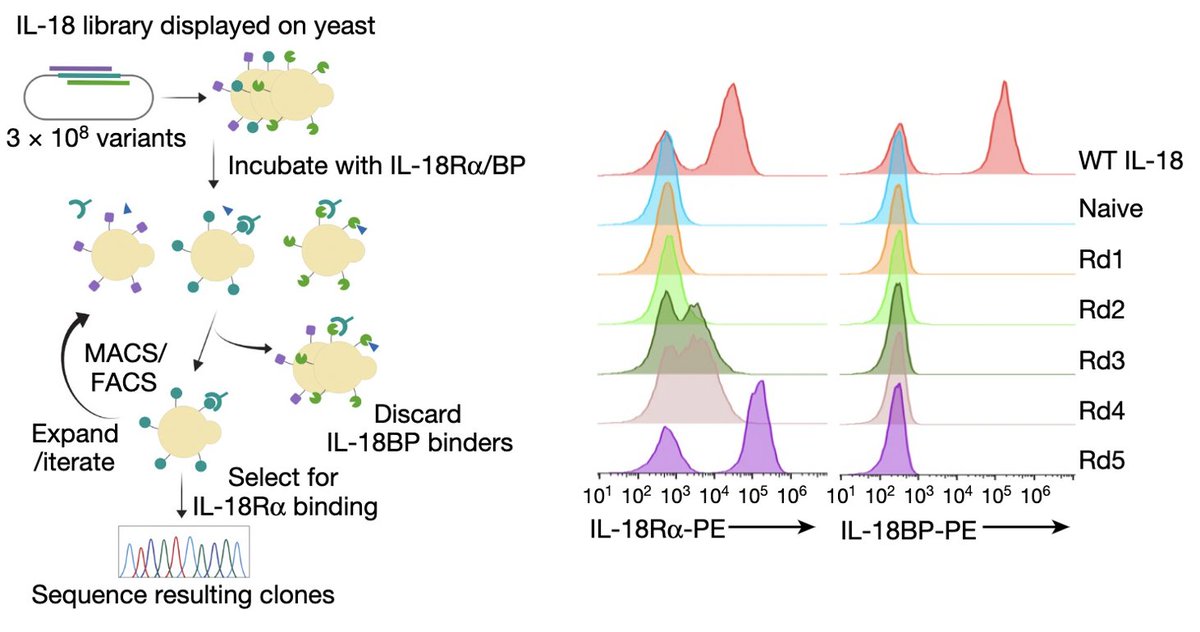
We thus set out to make a "decoy-resistant" version of IL-18 that we called DR-18. (11/)
We thus set out to make a "decoy-resistant" version of IL-18 that we called DR-18. (11/)
This was a tough engineering challenge because IL-18BP binds IL-18 ~10,000x stronger than IL-18Ra. Our solution to this problem was evolution: using yeast surface display, we screened ~300 million IL-18 muteins for those that only bound IL18Ra and avoided IL-18BP. (12/)
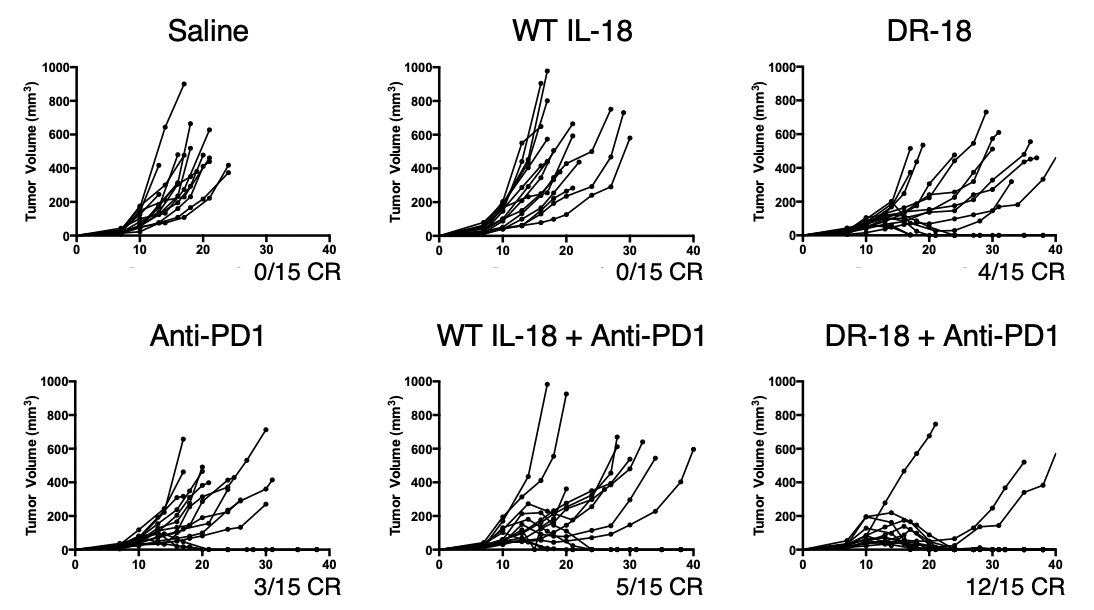
When we put DR-18 into mouse tumor models and compared it to WT IL-18, the results were dramatic. Just like in patients, WT IL-18 was entirely ineffective in mice. By contrast, DR-18 had single-agent activity that could clear established tumors and synergize with anti-PD1 (13/)
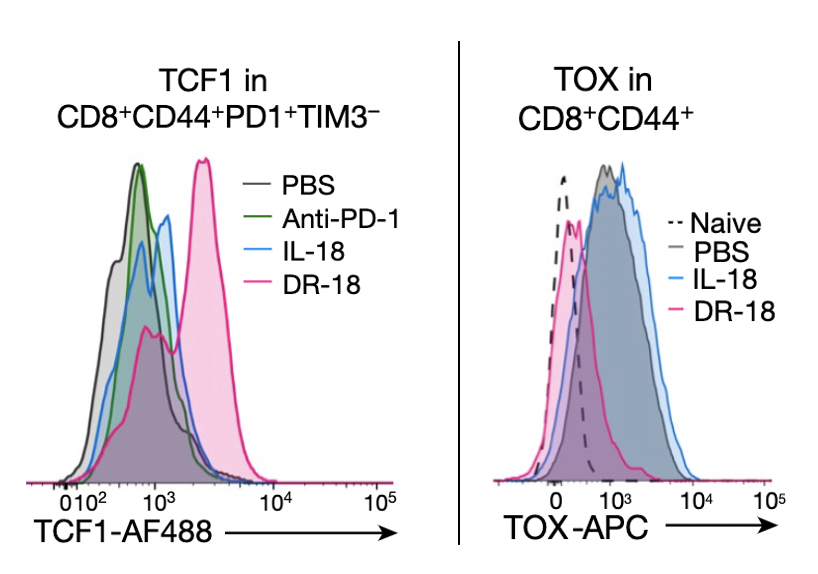
DR-18's MOA is pretty unique. To make a long story short, we found that it expands a key population of "stem-like" T cells that express TCF1 and skews their differentiation toward a highly active, polyfunctional state and away from an exhausted phenotype driven by TOX (14/)
Because IL-18 also can stimulate the innate immune system, we also wondered if it could act on tumors that were resistant to checkpoint inhibitors due to loss of MHC class I. The answer is yes, and in this case, its activity was NK cell mediated. (15/)
We are excited about the therapeutic potential of DR-18, and to that end, I recently started a company called Simcha Therapeutics to advance DR-18 into clinical trials. Right now, we are projecting the Phase I studies should begin in H1 of 2021. https://tinyurl.com/yare474u (16/)
This work was led by post-doc Ting Zhou @tz4st along with his co-first authors Orr-El Weizman @weizmano, and Bill Damsky @billdamsky. We also had major help from @MarcusBosenberg's lab- it was a consummate team effort. I'm so grateful to have been a part of this project. (17/)
I'm also grateful for the support of @IOTNmoonshot, Gabrielle's Angel Foundation @CureCancerNow, and the Blavatnik Fund for Innovation at Yale @YaleOCR for providing crucial funding for this program. (END)

 Read on Twitter
Read on Twitter