Arp2/3 and Mena/VASP Require Profilin 1 for Actin Network Assembly at the Leading Edge: A Thread
https://www.cell.com/current-biology/fulltext/S0960-9822(20)30633-3#.Xuyz-FaWRiQ.twitter
1/
https://www.cell.com/current-biology/fulltext/S0960-9822(20)30633-3#.Xuyz-FaWRiQ.twitter
1/
Our story begins when I was a post doc with @JamesZh80983643. We were studying how actin monomers were localized in neuronal cells during motility and axon guidance. Here is that paper:
https://www.cell.com/current-biology/fulltext/S0960-9822(13)00498-3?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS0960982213004983%3Fshowall%3Dtrue#.XvIJRS138yw.twitter
2/
https://www.cell.com/current-biology/fulltext/S0960-9822(13)00498-3?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS0960982213004983%3Fshowall%3Dtrue#.XvIJRS138yw.twitter
2/
We did shRNA knockdown of the monomer binding heavyweights Profilin 1 (PFN1) and Thymosin β4 (Tβ4) to see if they were involved in monomer localization to the leading edge. Surprisingly, it was Tβ4 that was largely responsible. 3/
However, knocking down PFN1 really did a number on the leading edge, which had way less actin and was much smaller than control cells. PFN1 was obviously important for actin assembly there. 4/
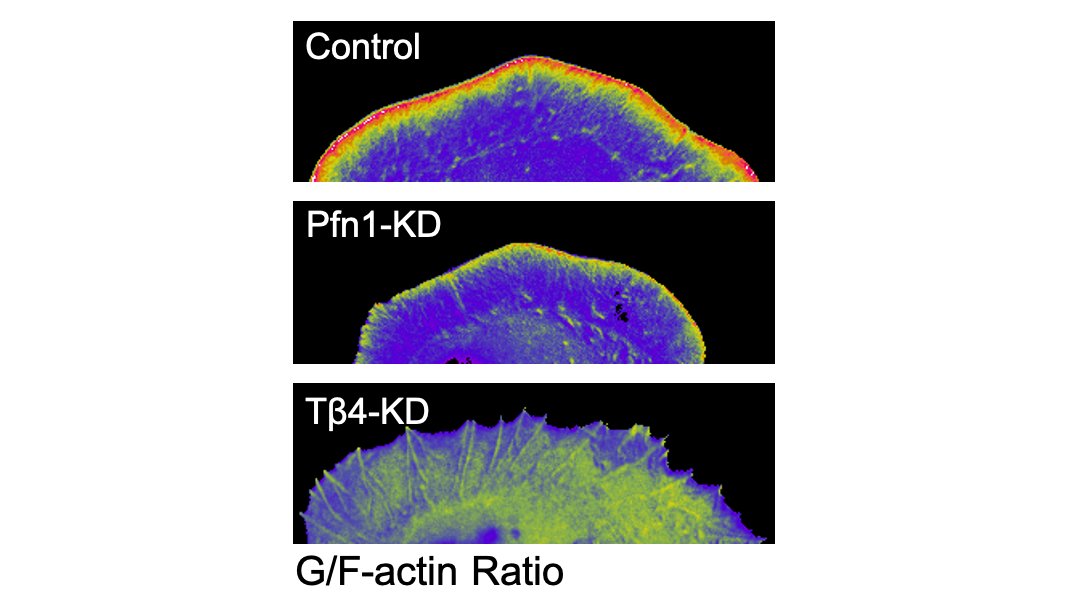
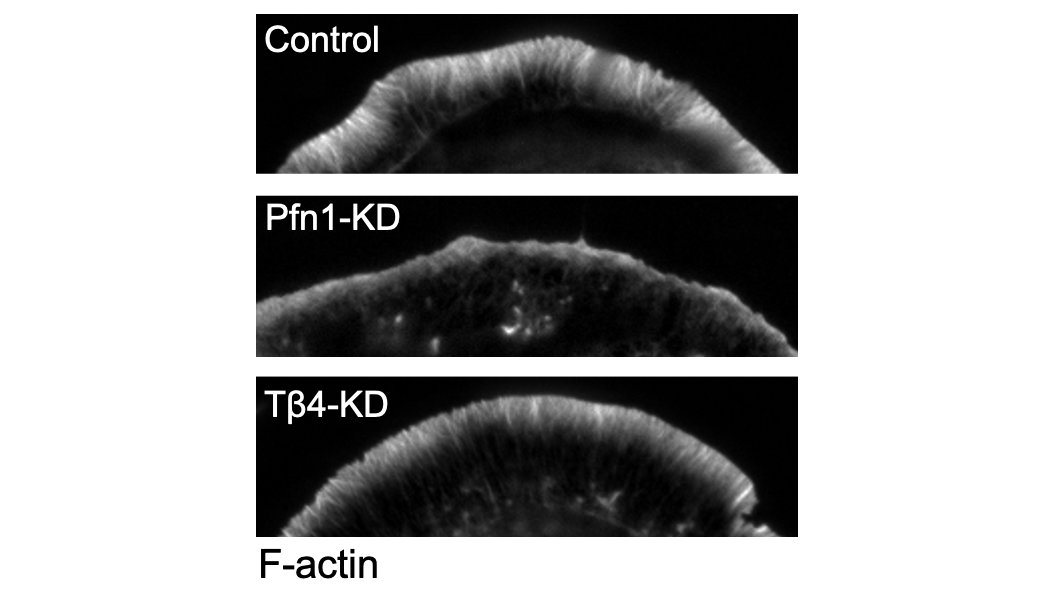
Here are some 3D-SIM images which show you just how different the actin looks when you knockdown PFN1. 5/
We followed up the first paper with a second where we followed the flux of actin monomers into the leading edge from different regions of the cell. Once again, Tβ4 was heavily important for this, so I put PFN1 aside for the time being.
https://www.cell.com/cell-reports/fulltext/S2211-1247(15)00302-2?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS2211124715003022%3Fshowall%3Dtrue#.XvIMmqupmN8.twitter
6/
https://www.cell.com/cell-reports/fulltext/S2211-1247(15)00302-2?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS2211124715003022%3Fshowall%3Dtrue#.XvIMmqupmN8.twitter
6/
Right as we were finishing up that paper, I became a PI. The first thing I wanted to do is study how PFN1 regulates the monomer pool to control what kinds of actin networks form at the leading the leading edge. 7/
One question that I have always been fascinated with is how monomers polymerize into some networks over others. Especially in complex structures, where competing networks overlap and are interdependent. You can imagine that even a small bias here could have a huge effect. 8/
My plan was to use CRISPR/Cas9 to make PFN1 KO cells so we can get a bona fide loss of function system. 9/
Right as I was planning to make the PFN1 KO cells, these two papers come out. They are awesome, and they directly tested some of the ideas I had. I remember thinking to myself, "what is there even left to do?"
https://www.cell.com/developmental-cell/fulltext/S1534-5807(14)00692-3?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS1534580714006923%3Fshowall%3Dtrue#.Xu0D3GO-NoM.twitter
https://www.cell.com/developmental-cell/fulltext/S1534-5807(14)00693-5?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS1534580714006935%3Fshowall%3Dtrue#.XvIRQ6uIc4Q.twitter
10/
https://www.cell.com/developmental-cell/fulltext/S1534-5807(14)00692-3?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS1534580714006923%3Fshowall%3Dtrue#.Xu0D3GO-NoM.twitter
https://www.cell.com/developmental-cell/fulltext/S1534-5807(14)00693-5?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS1534580714006935%3Fshowall%3Dtrue#.XvIRQ6uIc4Q.twitter
10/
Long story short, these papers beautifully demonstrate that profilin directs actin monomers away from Arp2/3 and towards formins and Mena/VASP-based filament networks. Elegantly shown with biochemical assays and experiments in yeast and mammalian cells. 11/
I decided to press on with the PFN1 KO cells, because I at least want to use them for knockout/rescue experiments to test how the ALS-linked PFN1 mutants are defective in regulating the cytoskeleton. 12/
Since I get asked this all the time, this thread seem like a good place to explain why we use CAD cells for these experiments. CAD cells are neuronal in origin and can differentiate into a neuron-like morphology when you remove serum from their growth medium. 13/
CAD cells also expressing neuron-specific genes like β3-tubulin. Because they can approximate the morphology and gene expression profile of neurons, they are a good model system to compliment studies in primary neurons or to study neuron-relevant cellular processes. 14/
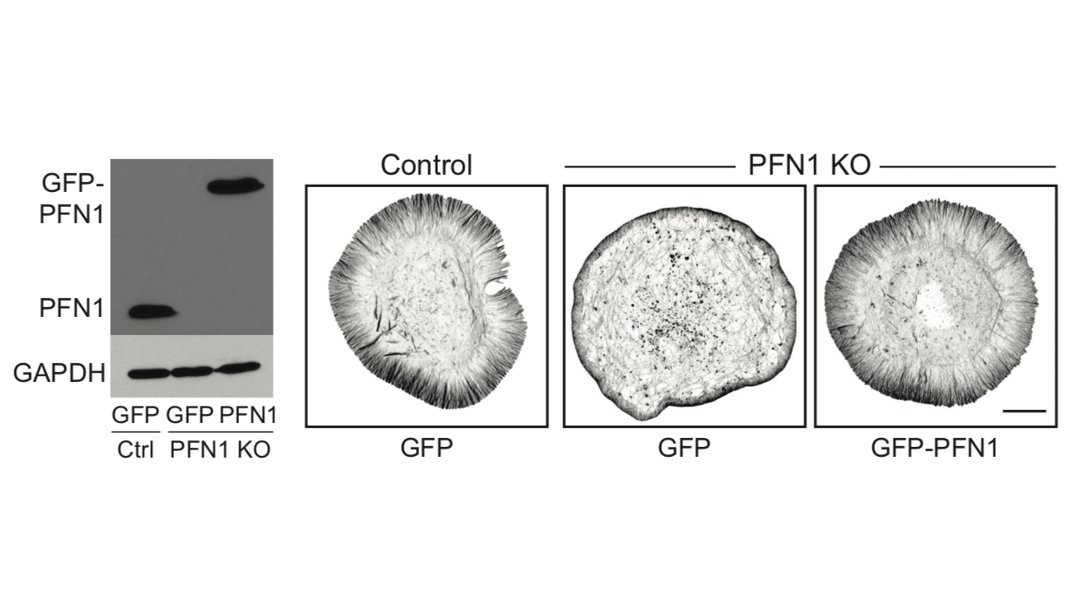
So we make the PFN1 KO CAD cells. They survive! We can also rescue them with physiological levels of GFP-PFN1 as a control. 15/
We did RNAseq (available in the paper) were pleased to see that very few genes for actin-binding proteins were differentially expressed in PFN1 KO cells. A clean system for understanding how PFN1 regulates actin! There were other surprises, but those are for another thread. 16/
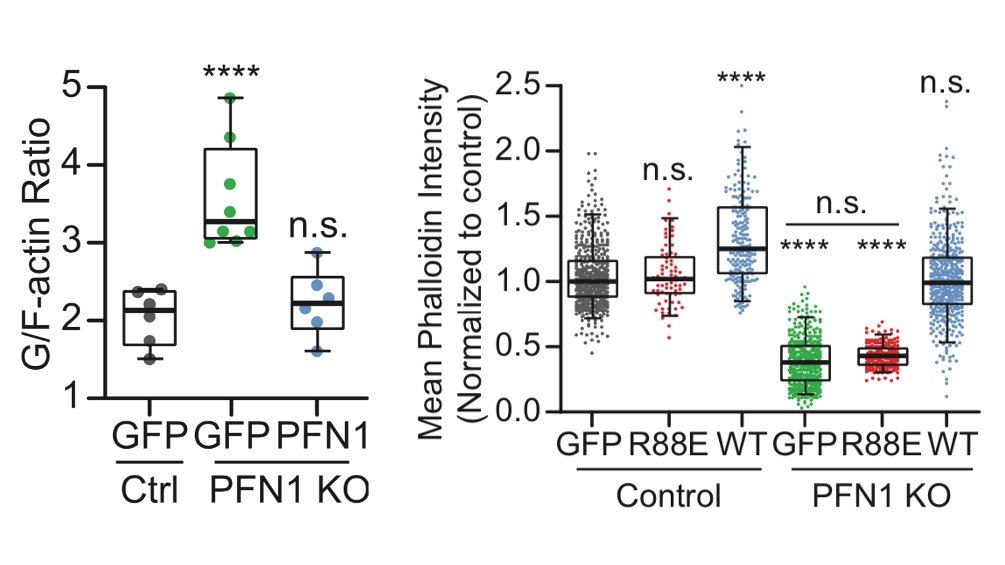
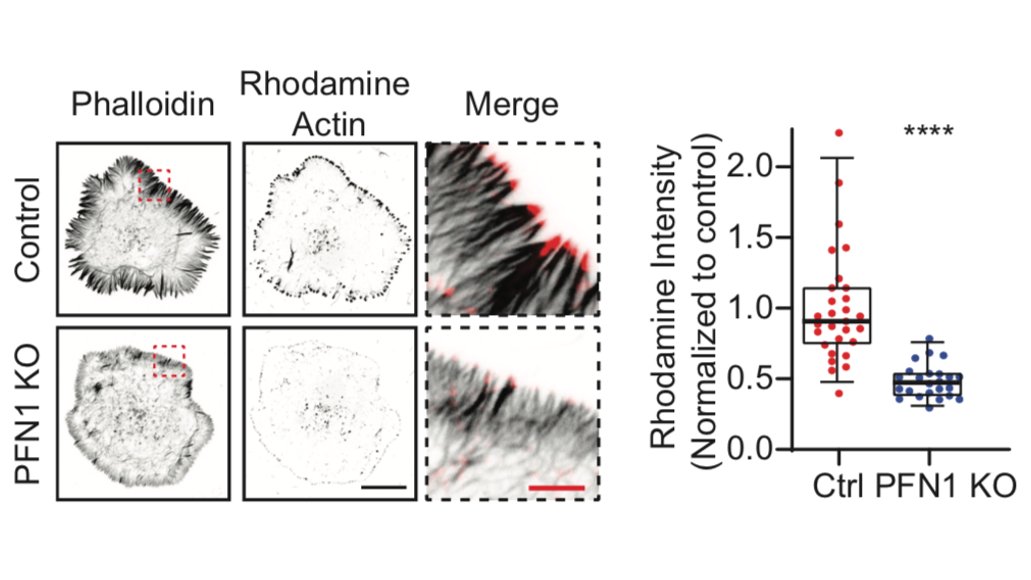
The first result which shocked me is how much less polymerized actin there was in these cells. PFN1 KO cells have >60% less F-actin. The actin just sits in the monomer pool, unable to assemble. 17/
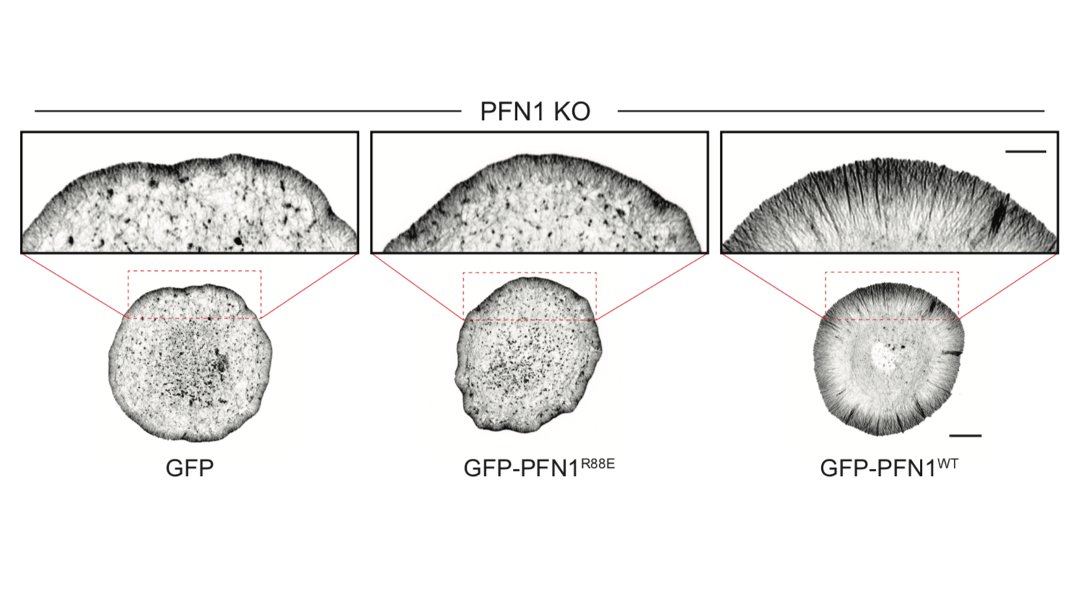
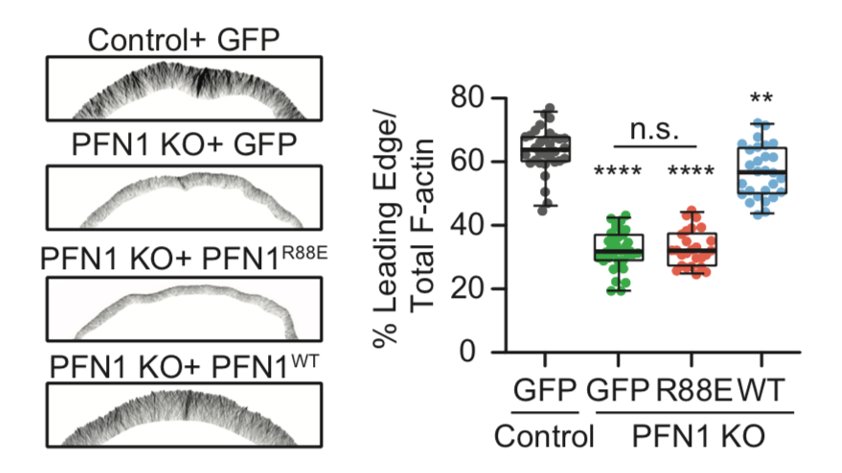
The biggest effects were are the leading edge, though, so we focused our attention there. FYI, the R88E mutant shown below is actin-binding deficient. 18/
There is also fewer polymerizing filaments, shown here with the classic barbed-end labeling assay. I have begun to wonder if this assay labels all polymerizing filaments or if there is some bias, but that is also probably for another thread. 20/
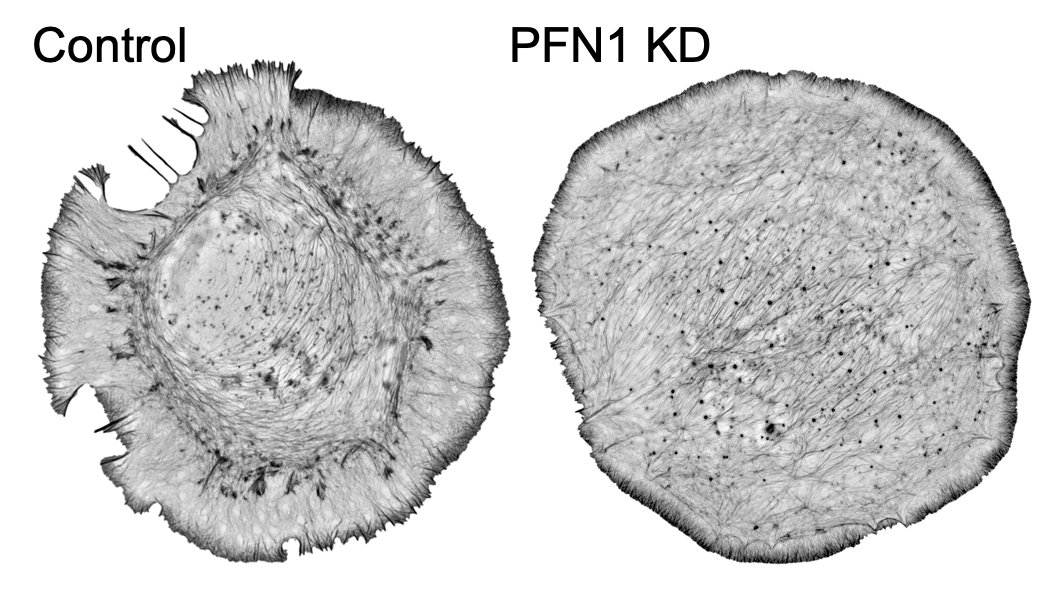
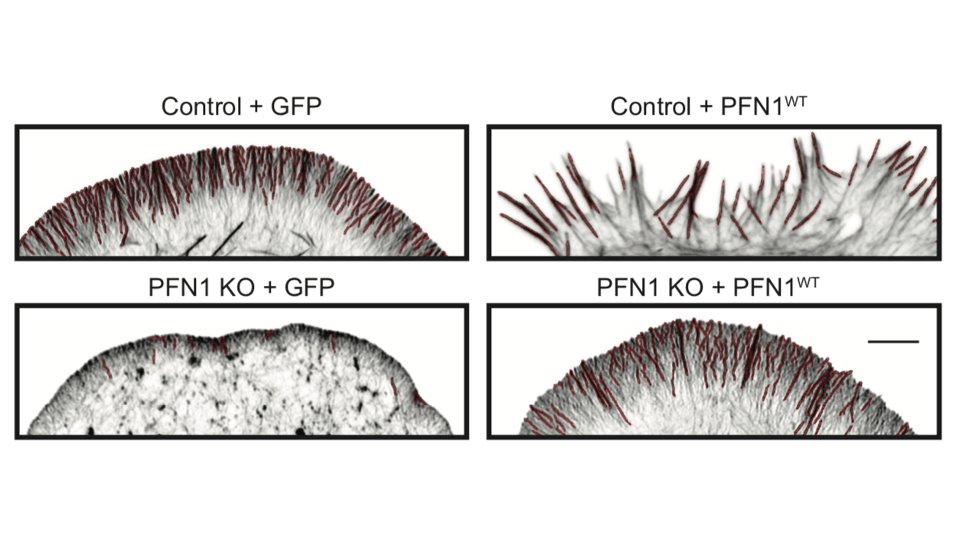
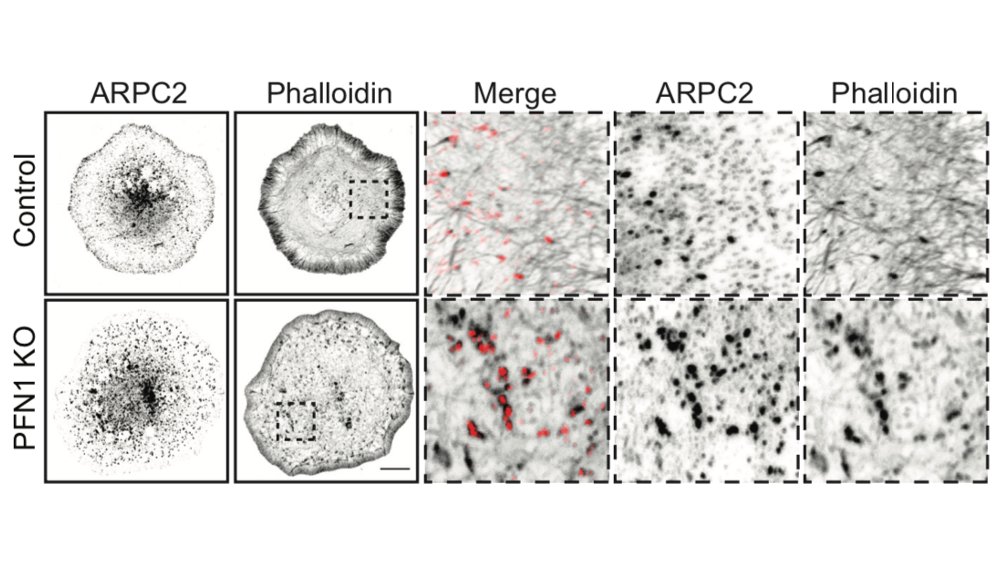
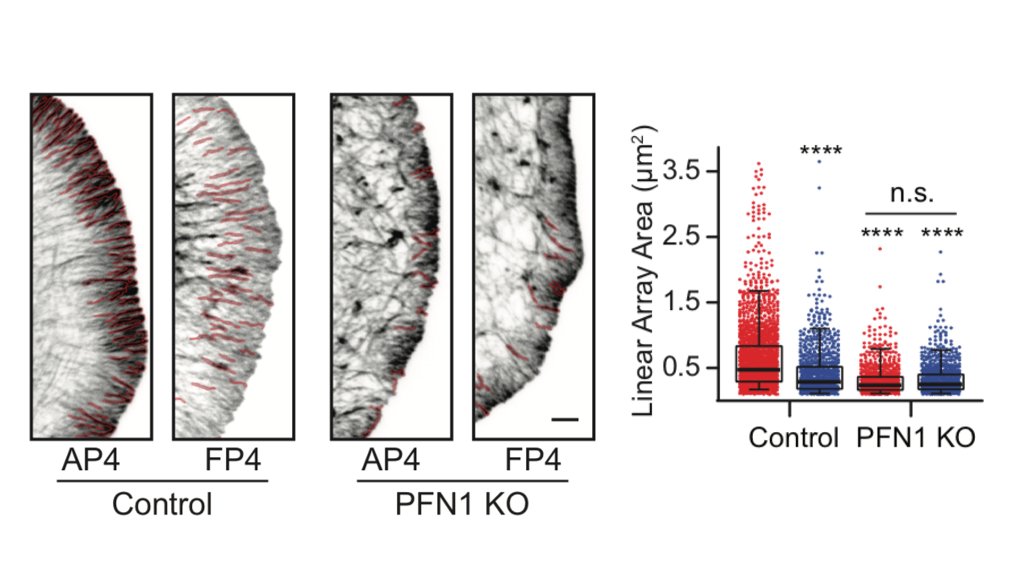
But it's not just the amount of actin at the leading edge that is different, but also the filament architecture. 21/
Why are there structures labeled in red in the image above? We used an ImageJ plugin called Tubeness, originally designed to measure mitochondria morphology in yeast (I believe), to segment the linear, bundled actin structures.
https://www.longair.net/edinburgh/imagej/tubeness/
22/
https://www.longair.net/edinburgh/imagej/tubeness/
22/
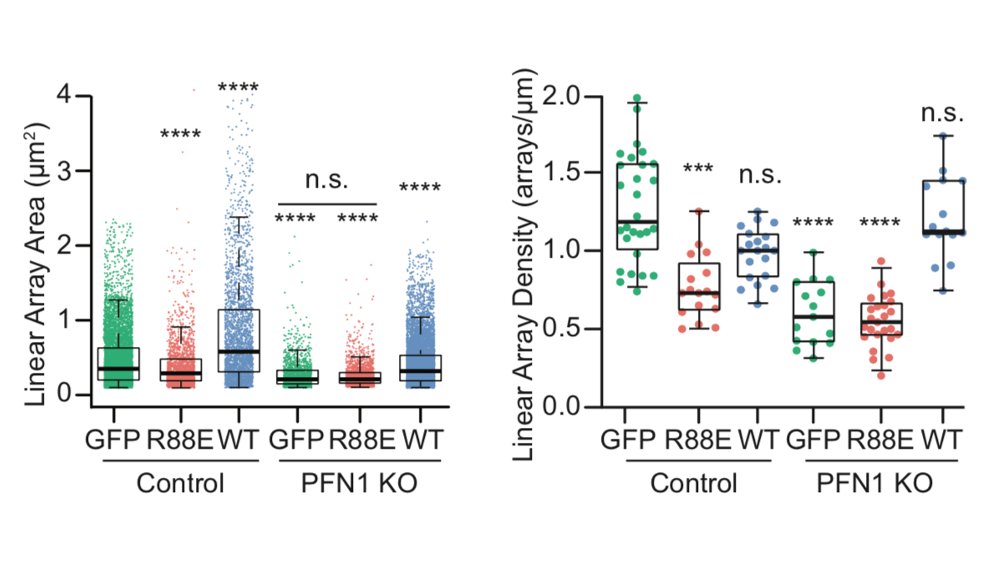
I wasn't sure that it was going to work, but it worked extremely well! We used it to measure the area of individual linear arrays, as well as their density at the leading edge. Huge loss of both in PFN1 KO cells. 23/
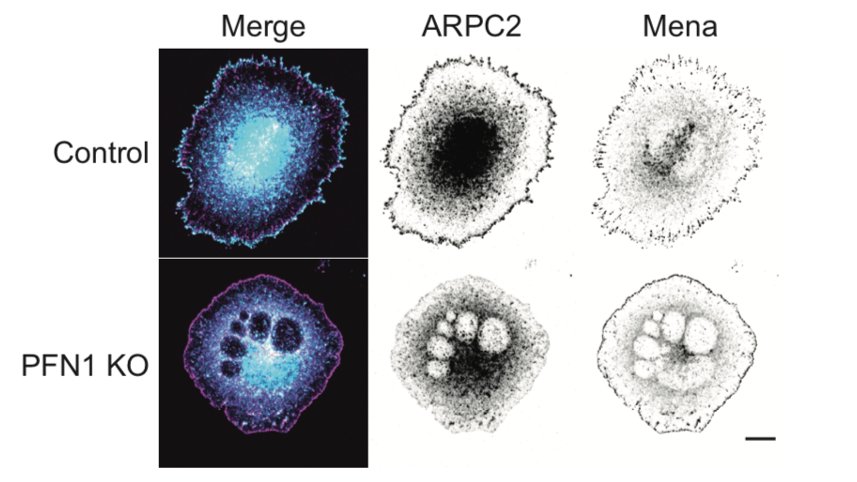
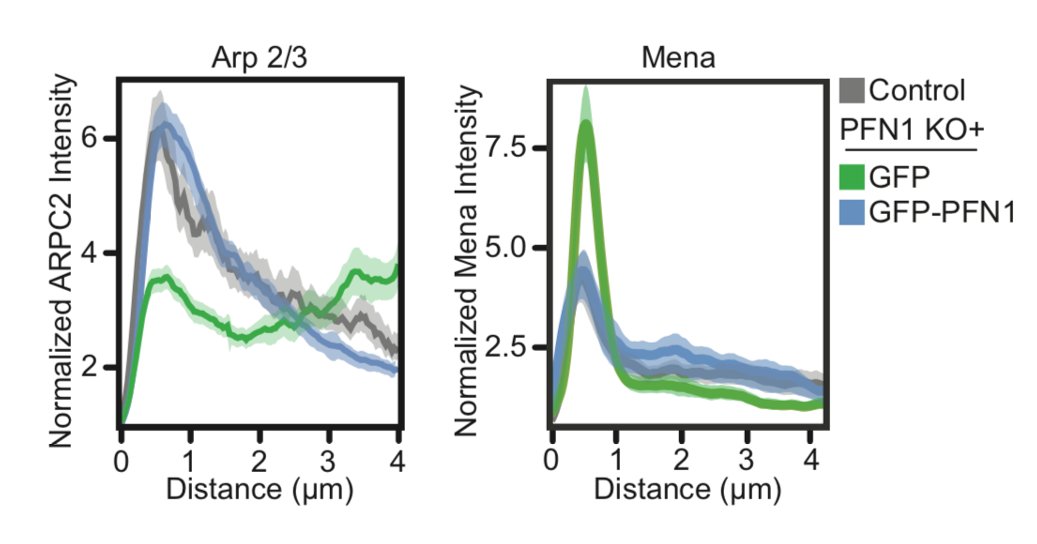
Because leading edge architecture was so affected by loss of PFN1, the natural next step was to look at Arp2/3 and Mena/VASP-dependent actin assembly. The papers I linked earlier would predict that Arp2/3 networks would be increased and Mena/VASP decreased in PFN1 KO cells. 24/
There is where things got a little wacky. Instead of enhanced Arp2/3 at the leading edge of PFN1 KO cells, we saw almost none! 25/
Seriously- practically none at all. Also, Mena localization was enhanced at the leading edge in PFN1 KO cells. What is going on here? Now the fun was really starting to begin. 26/
Let's start with Arp2/3. While not at the leading edge, it did co-localize with actin puncta in the cell center, which PFN1 KO cells had a lot of. Inhibiting Arp2/3 got rid of these puncta. Thus, there is still PFN1-independent Arp2/3 assembly, just not at the leading edge. 27/
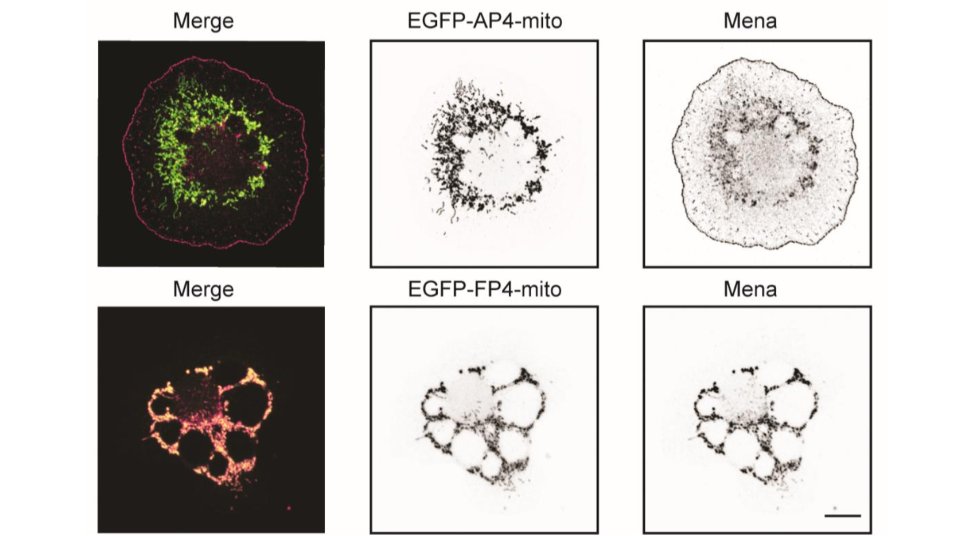
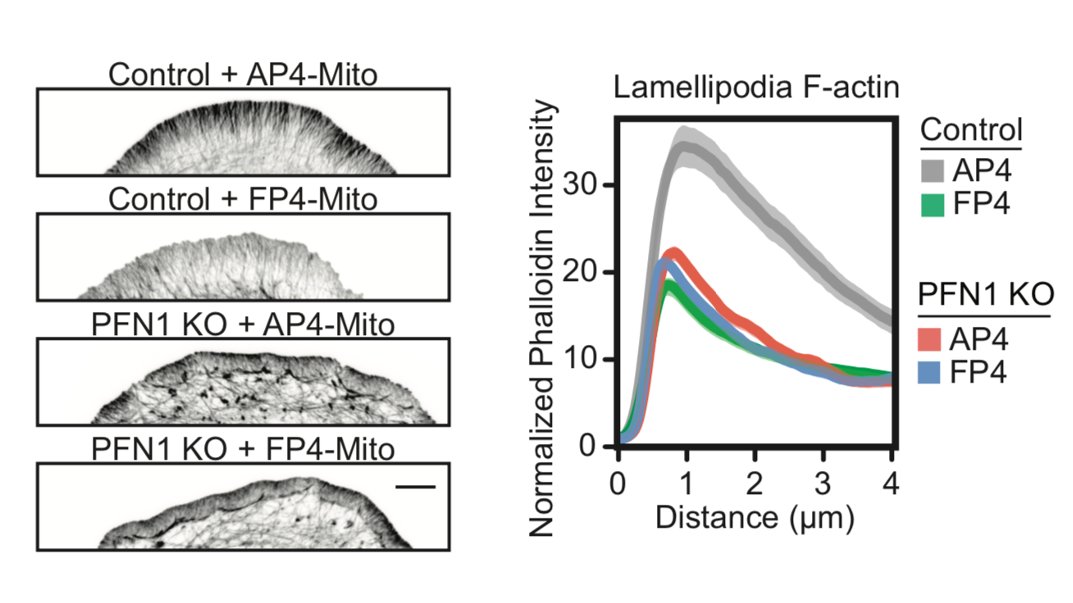
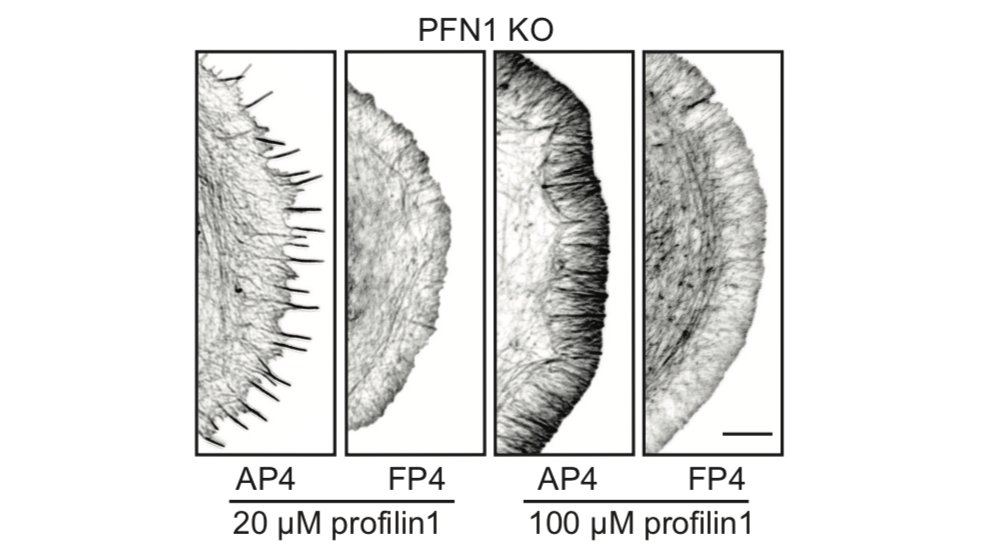
Now Mena/VASP. Even though it is at the edge, it doesn't appear to be able to polymerize actin at all. I will show a couple of examples, but there are more in the paper. We used the FP4-mito construct to target Mena/VASP to mitochondria. AP4-mito is the control. 28/
Here is the effect of Mena/VASP inhibition on linear filament arrays. Their assembly is dependent on Mena/VASP (see Control cells), and they are dramatically reduced in PFN1 KO cells. However, sequestering Mena/VASP in PFN1 KO cells does nothing. 29/
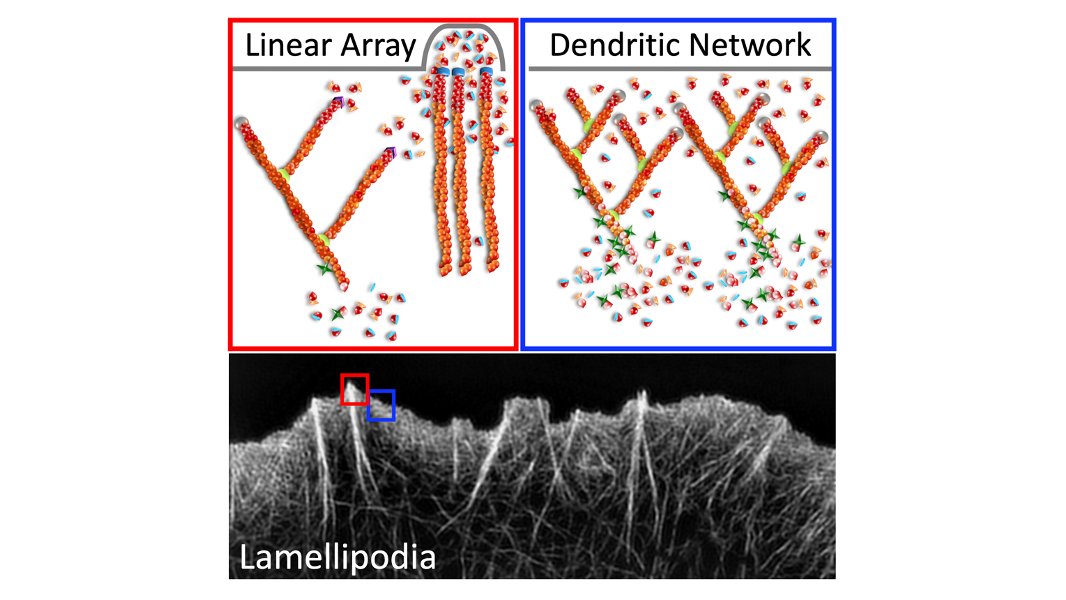
Now let's look at the lamellipodia. When I say this, I mean the actin structures that you cannot resolve by light microscopy, in between the linear arrays. The lamellipodia is also largely dependent on Mena/VASP proteins, but inhibiting them in PFN1 KO cells does nothing. 30/
There has been some back-and-forth in the literature on whether Mena/VASP proteins require profilin-actin to polymerize filaments, or if it just enhances their activity. In our hands Mena/VASP appears to be completely inactive without PFN1. Many more examples in the paper. 31/
Now, the story is nice but it feels like we are missing something. I keep thinking about Arp2/3-Mena/VASP competition, which is actually the product of many different variables (concentration, affinity, etc.). 32/
I have also always admired biochemists. They often derive the best functional information because they are able to carefully control reaction components. This is very tough in cell biology, where usually it's all or nothing and you have to infer what is happening in between. 33/
I have always wanted to do something similar in cell biology experiments. But how can you accurately control protein concentration in a cell? Then I read this paper from the Watanabe group and my jaw fell to the floor:
https://www.molbiolcell.org/doi/full/10.1091/mbc.e13-03-0162#.Xu0E0WxoygI.twitter
34/
https://www.molbiolcell.org/doi/full/10.1091/mbc.e13-03-0162#.Xu0E0WxoygI.twitter
34/
Here, they used the Invitrogen Neon electroporator to introduce fluorescently labeled actin into cells at single-molecule levels. Very interesting! Cell transfection was high, and they were able to reproducibly get low concentrations of actin into cells. 35/
Backstory time! When I was a graduate student in the Hahn Lab at UNC-CH, they were still developing protein-based biosensors. Some of these had amazing properties, but were not as widely used because they had to be microinjected into cells. 36/
So, even though they were in some ways inferior to their purified protein-based counterparts, genetically encoded biosensors had much broader appeal. 37/
However, from time to time, the lab would try out whatever new product was on the market to see if it could reliably get protein into cells. Nothing really worked that well. Toxicity, low transfection rates, and variable amounts of protein into each cell. 38/
Microinjection works great, but is an acquired skill. Also, there are two issues. One is that you can only inject maybe 50-100 cells at a time. And if you are really good only 10-20% of those will die. 39/
The other issue with microinjection is that you never know how much protein actually gets into the cell. Just the needle concentration. From cell to cell there can be considerable variability. 40/
Any way, long after I had given up on being able reliably get protein into cells, I am reading the Watanabe paper and seeing their awesome electroporation results with the Neon. Is this the lost city of Atlantis that we had always been looking for??? 41/
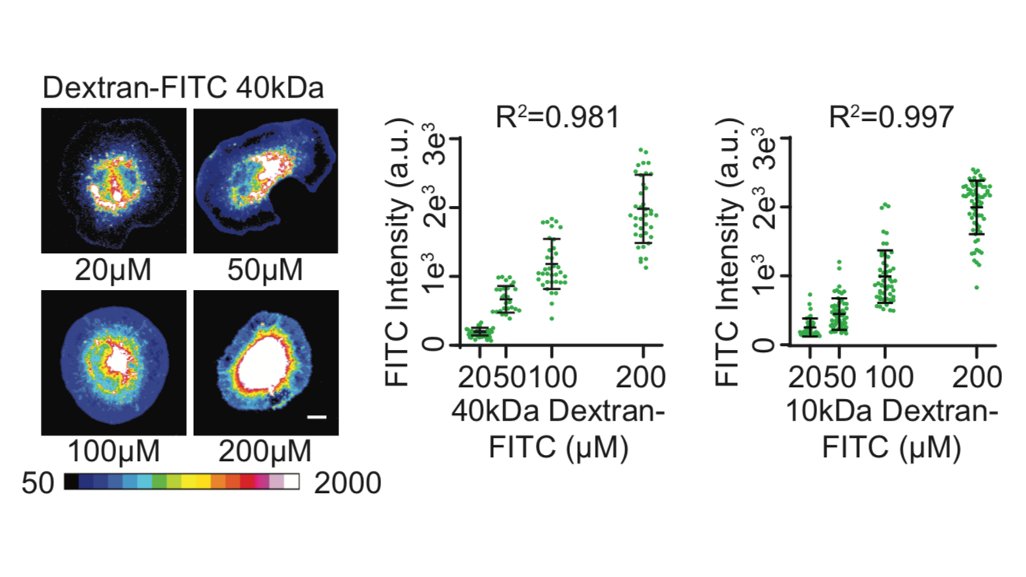
I order a demo. For this to work like I want it to, the amount of material that gets into the cell has to be linearly proportional to the bath concentration in the electroporation reaction. We used two different sizes of fluorescently-labeled Dextran to test it out...42/
It works! UNBELIEVABLE!! Linearly proportional to the bath concentration. Also, transfection rate is >99%, you can do a million cells at a time, and discrete steps can be seen between the different concentrations. 43/
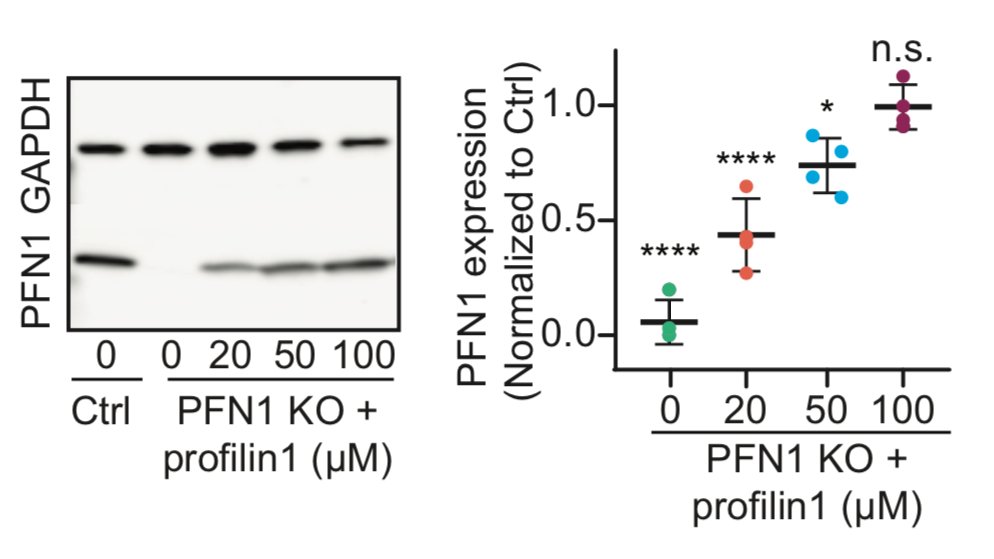
It also worked beautifully for PFN1 protein. We can introduce PFN1 all the way to physiological levels, with discrete steps in between. Now it's time to party and look at concentration-dependent effects of PFN1 on the leading edge! 44/
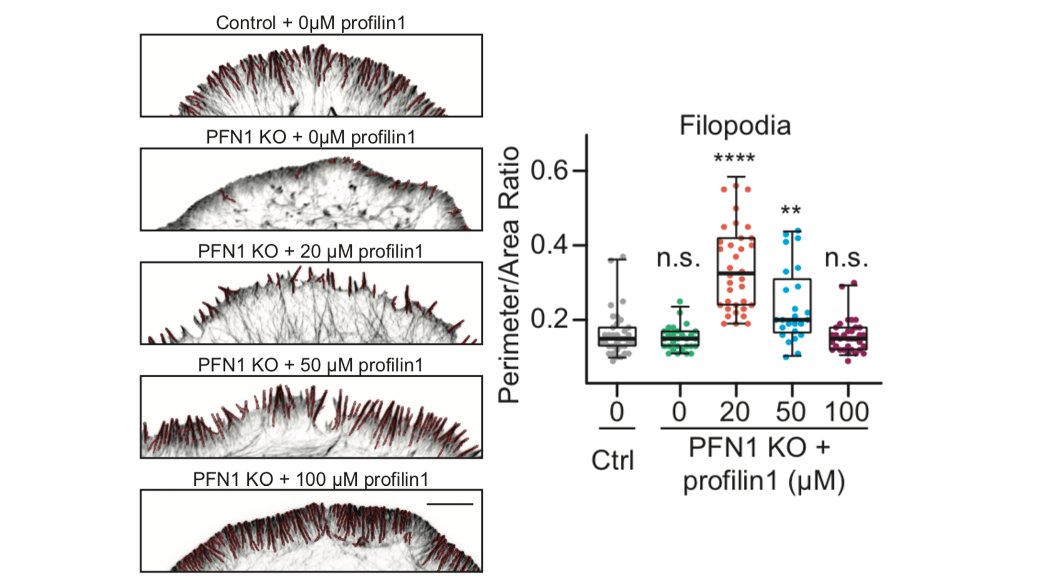
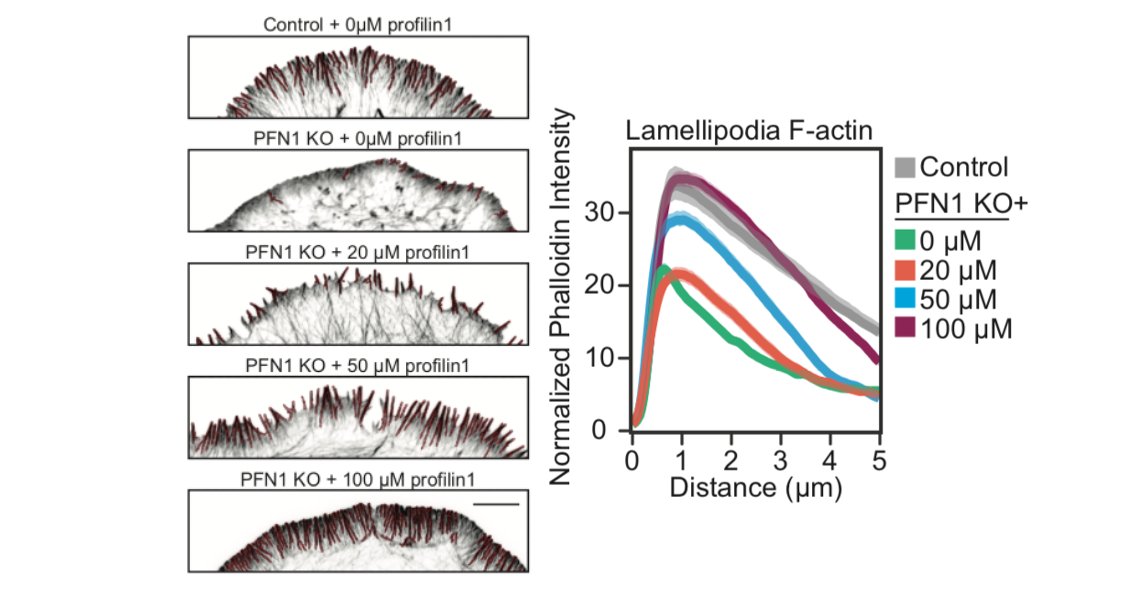
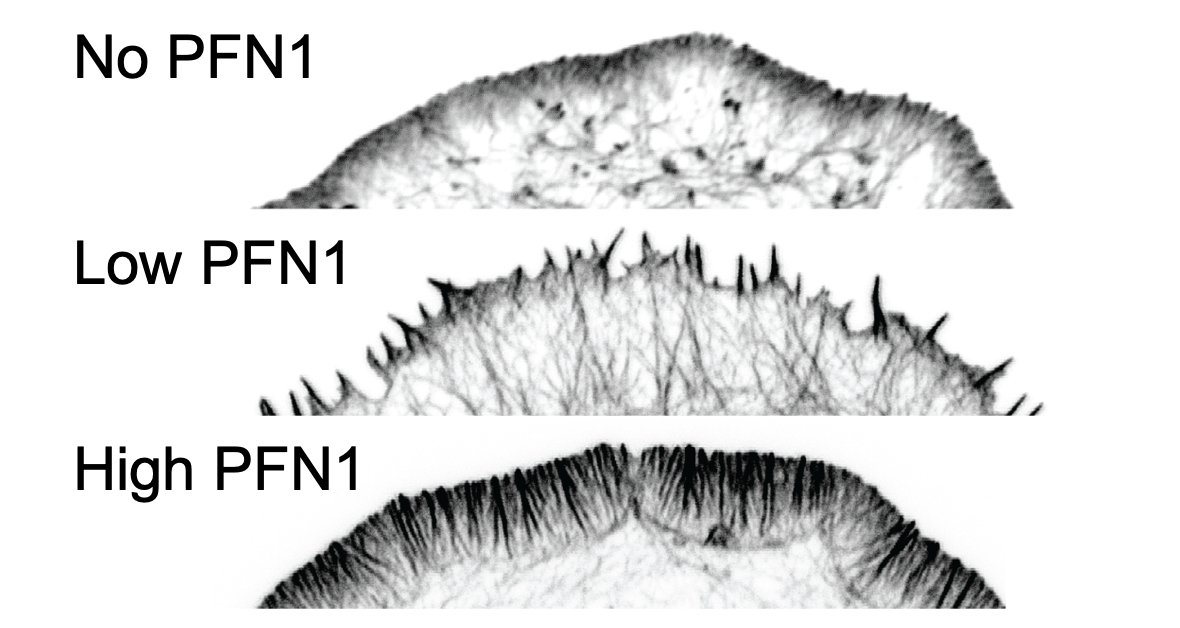
Here is the effect of PFN1 concentration on lamellipodia. Steady increase with increasing PFN1 levels. 46/
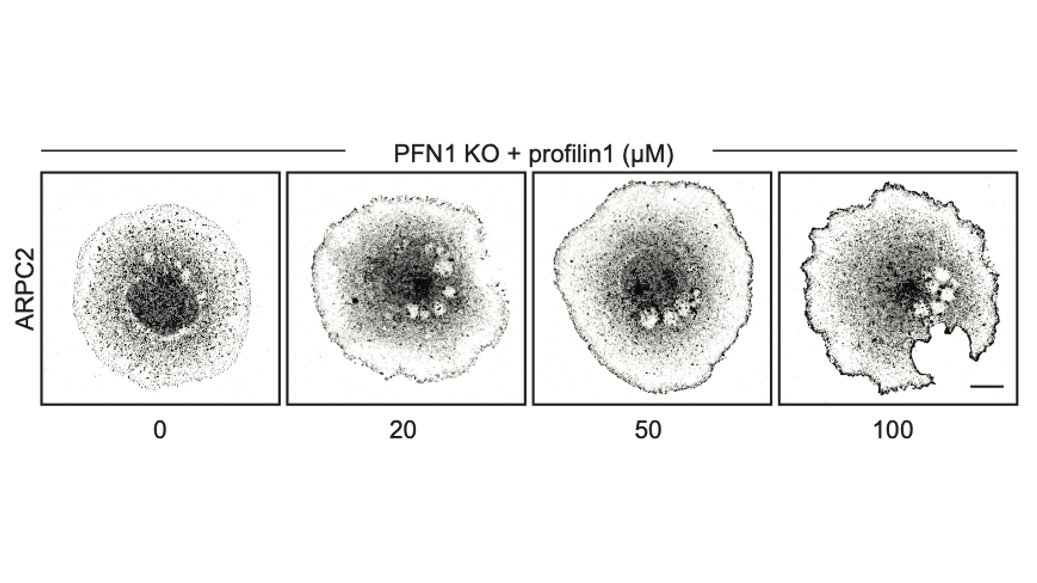
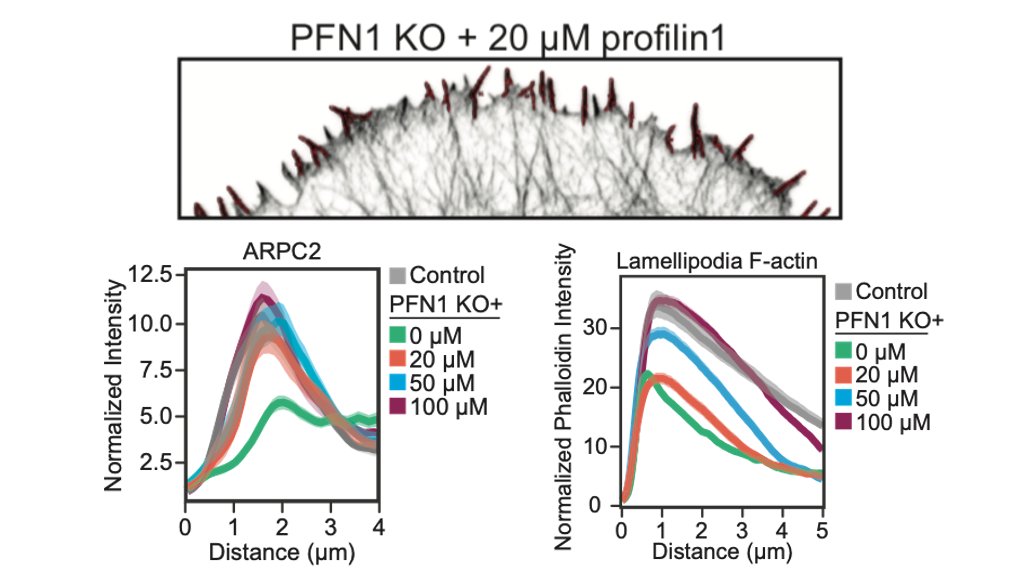
Here is the effect of PFN1 concentration on Arp2/3 localization. Also increases with PFN1 levels, but even the lowest concentration of PFN1 restores most Arp2/3 re-localization to the leading edge. 47/
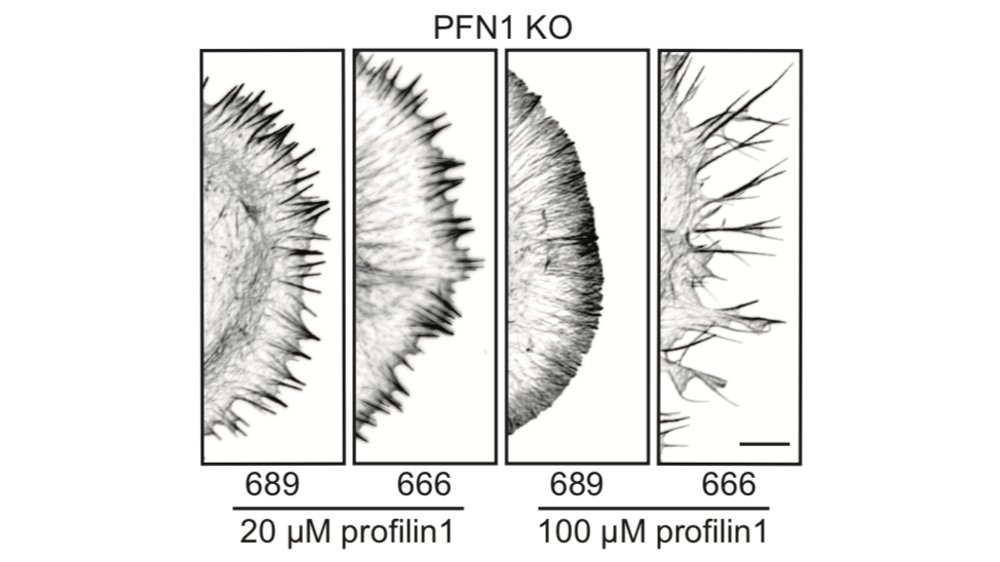
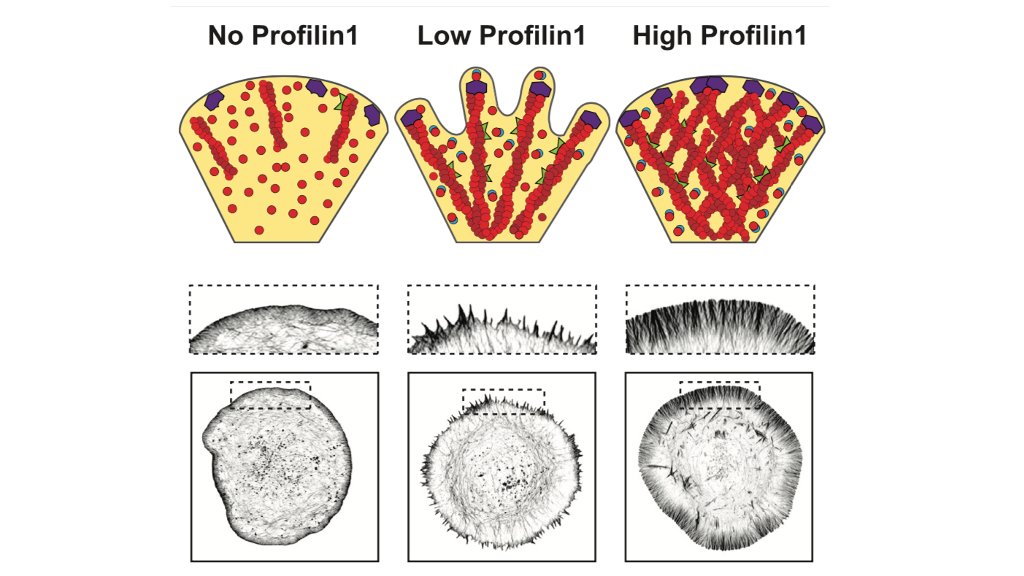
That low concentration of PFN1 was the most interesting. Here you have only filopodia and no lamellipodia, despite a considerable amount of Arp2/3 re-localizing to the leading edge. At low PFN1, Mena/VASP is competing with Arp2/3 networks for PFN1-actin and winning. 48/
And yes, we did the experiment to determine if the filopodia that you see at low PFN1 concentrations are Mena/VASP-dependent. SPOILER ALERT: they are. 49/
So, we are able to see Mena/VASP and Arp2/3 competition at low PFN1 concentrations. What happens at normal levels? Here, there is enough PFN1-actin for everybody and lamellipodia and linear arrays co-exist at the leading edge. There are no filopodia at all, though. 50/
This is probably because Arp2/3 networks inhibit their formation once they get going. Here is what happens when you inhibit Arp2/3 in the presence of a normal dose of PFN1 protein. Filopodia explode from the cell edge. 51/
Also note that Arp2/3 isn't needed for filopodia to to form at low PFN1 concentrations. Thus, they are not likely to be the result of convergent elongation.
https://doi.org/10.4161/cam.5.5.16971
52/
https://doi.org/10.4161/cam.5.5.16971
52/
So, in addition to showing that Arp2/3 and Mena/VASP networks require PFN1 to assemble actin at the leading edge, we also show that there are discrete stages of internetwork competition and collaboration between these networks that occur at different PFN1 expression levels. 53/
It was very interesting to see that Mena/VASP assembly was needed for Arp2/3 to localize to the leading edge. This was reminiscent of the filopodia/veil model that was first described in growth cones. 54/ https://jcs.biologists.org/content/120/6/1113
I should also say that the requirement of Mena/VASP assembly for Arp2/3 leading edge localization is likely to be cell-type dependent. It may be restricted to cells with high profilin, Mena/VASP. 55/
You should also check out the most recent paper from Jan Faix's lab where they look at the effect of complete loss of Mena/VASP proteins on the lamellipodia. 56/ https://doi.org/10.7554/eLife.55351
Without Mena/VASP, they see dramatic reduction in leading edge actin. Enhanced Arp2/3 localization, though. But maybe not completely effective? It would be interesting to determine what is different between their cells and our PFN1 KOs. 57/
This make me really wish we labeled Arp2/3 in control cells treated with FP4-mito. I will still probably do the experiment just to satisfy my own curiosity. 58/
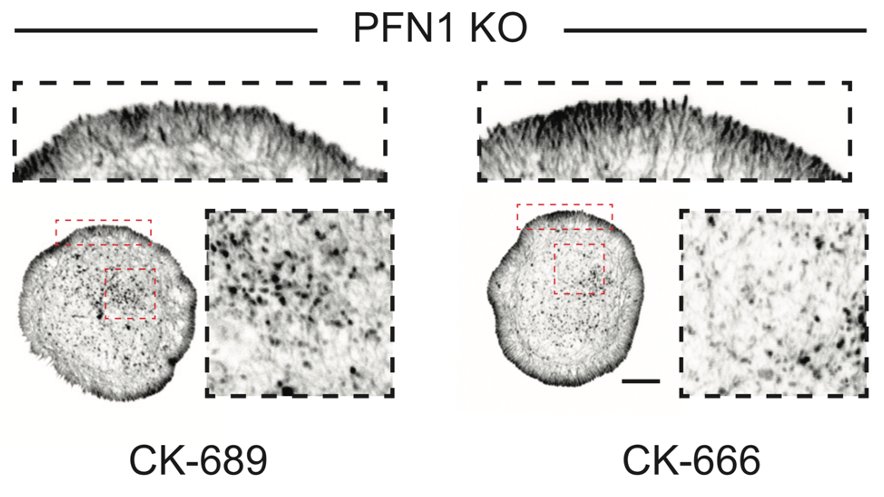
Also, I really want to do EM on CK-666 treated PFN1 KO cells. Where are those actin filaments coming from? Arp2/3 is not at the edge, and the inhibitor should have knocked out any residual activity there. Not Mena/VASP either...59/
Details aside, I think the biggest result is that PFN1 knockdown ≠ PFN1 knockout. There were very different effects from partial and complete loss of PFN1. And this could be true for many proteins. It will be important to examine this more closely in the future. 60/
For example, does PFN1 promote or inhibit filopodia formation? The answer is both- it depends on where you start. What if the experiment was performed with only two points? Either result could have been concluded. 61/
Now I need to acknowledge @kskruber1, who did nearly all of the experiments for this paper. The word rock star gets thrown around a lot, but Kristen is the real deal. She POWERS through the most difficult experiments and analysis. And her enthusiasm for science is infectious. 62/
@kskruber1 also had some help with image analysis from undergraduate extraordinaire @peytonwarp. Peyton is going big places, I can promise you that!
Also a huge shoutout to @cytoskeletown. Without Jessica, the grand finale experiment wouldn't have been possible. If she isn't one of your scientist buddies, you're doing it wrong! 63/
Also thanks to our other collaborators who aren't on Twitter: James Thomas, Maury Swanson, Tracy-Ann Read. You should jump on here, because you deserve the @'s. 64/
If anyone is doing this paper for an online journal club, let me know and me and/or @kskruber1 will try to jump on at the end to answer questions. It also makes a great talk (hint, hint!). 65/AND DONE. Phew!

 Read on Twitter
Read on Twitter