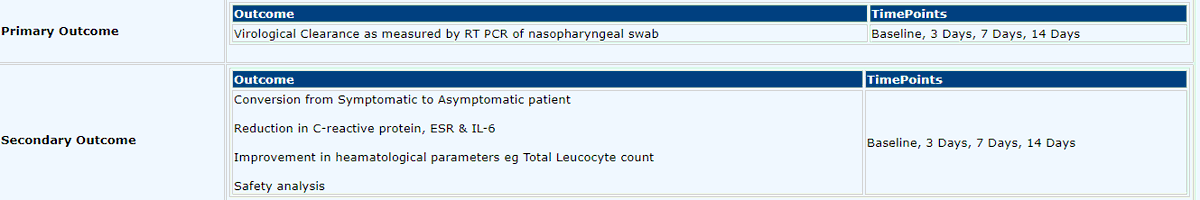Statement issued by the Ministry of Ayush on claims of Patanjali Ayurved regarding treatment of COVID-19
Related to this, the only information available in the public domain is this CTRI registration
http://ctri.nic.in/Clinicaltrials/showallp.php?mid1=43900&EncHid=&userName=patanjali
http://ctri.nic.in/Clinicaltrials/showallp.php?mid1=43900&EncHid=&userName=patanjali
This trial was registered on CTRI on 20 May 2020, sponsored by Patanjali.
Trial conducted at National Institute of Medical Sciences Jaipur https://nationalinstituteofmedicalsciences.com/
Seemingly a private medical college in Jaipur
Apparently approved by the local IEC
Trial conducted at National Institute of Medical Sciences Jaipur https://nationalinstituteofmedicalsciences.com/
Seemingly a private medical college in Jaipur
Apparently approved by the local IEC
This 'prospective' study was supposed to recruit 120 trial participants
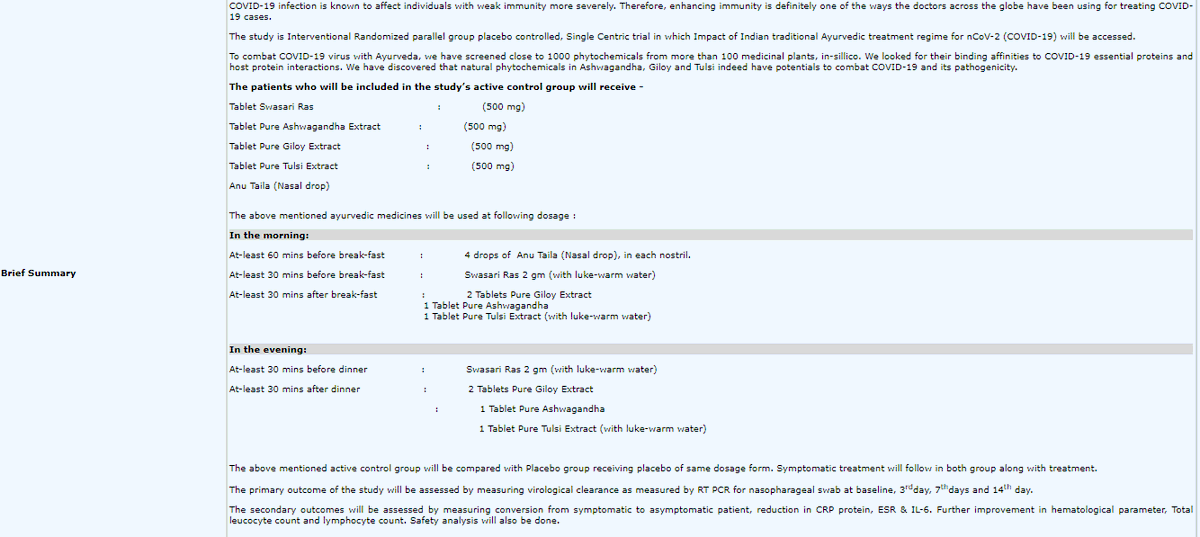
Apparently this is what constitutes Coronil! These seem to be established Ayurvedic medications given together. Is that package what is called Coronil? Or they have mixed up the ingredients of all these into a new packaged drug called Coronil?
Has anyone seen trial results published anywhere? Not me at least?
What was given to the control arm?
What was given to the control arm?
Was it just a placebo? Or did they get current standard of care (supportive therapy) for Covid-19 or not (for the patients who needed therapy)
If not, is that not a major ethical issue (actually not giving it to intervention arm would also be one)
If not, is that not a major ethical issue (actually not giving it to intervention arm would also be one)
what was the nature of 'informed consent'?
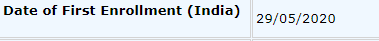
Date of first enrolment was 29 May 2020, the trial is still enrolling as per the CTRI link as of today
First patient enrolled on 29 May 2020, outcomes need to be measured for at least 14 days after beginning therapy.
And on 23 June 2020, in a trial which is still recruiting, not yet published, they announced efficacy via press conference?!
And on 23 June 2020, in a trial which is still recruiting, not yet published, they announced efficacy via press conference?!
Incidentally, the ethics committee of this institution -National Institute of Medical Sciences and Research-does not seem to be registered (or re-registered) with CDSCO/DCGI
https://cdsco.gov.in/opencms/opencms/en/Clinical-Trial/Ethics-Committee/
https://cdsco.gov.in/opencms/opencms/en/Clinical-Trial/Ethics-Committee/
Has anyone seen any data on pre-clinical studies, safety studies?
If they are using existing medications and packaging these, has this been mentioned anywhere?
Is Coronil just a package of existing Ayurvedic medications as the trial entry seems to indicate?
If they are using existing medications and packaging these, has this been mentioned anywhere?
Is Coronil just a package of existing Ayurvedic medications as the trial entry seems to indicate?
Now this update. The clinical trial investigators claim that they informed CCRAS @moayush on 2nd June about their study
Prof @spkalantri questions the end points used in the study https://twitter.com/spkalantri/status/1275633747614982144
Our discussion on this and other trial related issues yesterday evening https://twitter.com/AnantBhan/status/1275659349730476035
Quoted in this story by @ChandnaHimani https://twitter.com/sandygrains/status/1275695092213387268
Gets murkier https://twitter.com/ANI/status/1275705563641454592

 Read on Twitter
Read on Twitter