Thread/
There are many new exciting trials in transthyretin #amyloidosis – as we continue to enroll patients in the CARDIO-TTRansform trial, I thought I would highlight some of these trials in ATTR-CM
https://clinicaltrials.gov/ct2/show/NCT04136171
#cardiotwitter
There are many new exciting trials in transthyretin #amyloidosis – as we continue to enroll patients in the CARDIO-TTRansform trial, I thought I would highlight some of these trials in ATTR-CM
https://clinicaltrials.gov/ct2/show/NCT04136171
#cardiotwitter
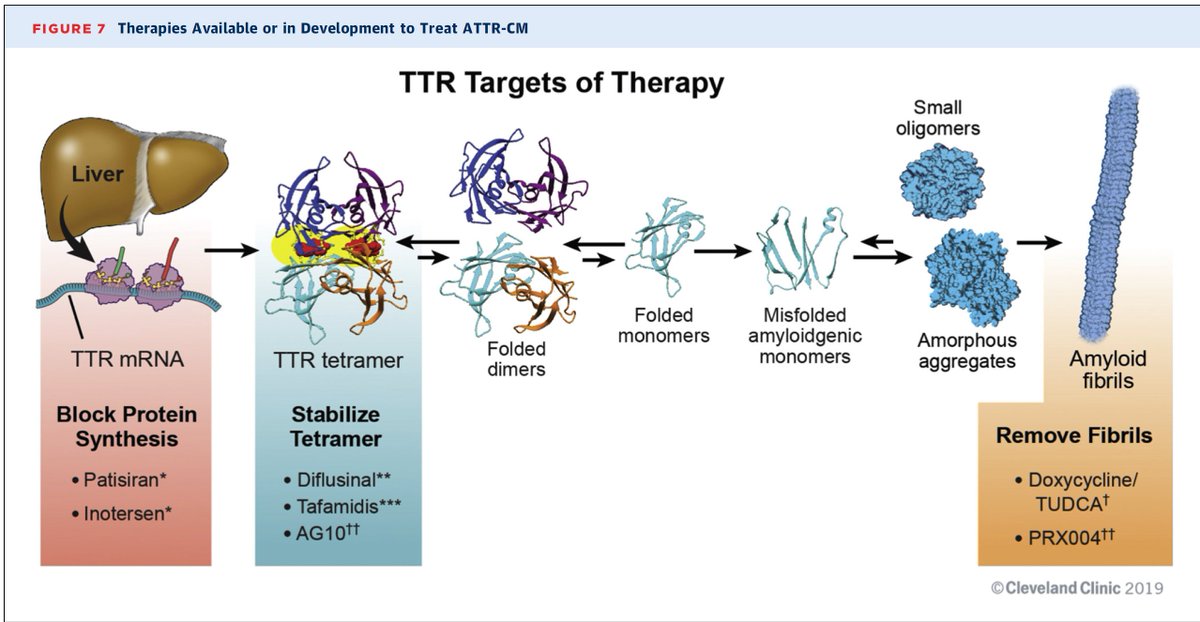
If you recall, 2 main approaches to treating ATTR #amyloidosis with approved drugs (figure credit @frederickruberg @maz_hanna )
1)Stabilizers: bind to the TTR tetramer to stabilize it. #tafamidis , AG-10, diflunisal
2)Silencers: Inotersen and Patisiran
1)Stabilizers: bind to the TTR tetramer to stabilize it. #tafamidis , AG-10, diflunisal
2)Silencers: Inotersen and Patisiran
tafamidis is prescribed to ATTR-CM pts & inotersen/patisiran for those with hATTR polyneuropathy
But – 2 main questions in ATTR-CM
1)Which is associated with better outcomes – silencer or stabilizer?
2)Is the combination of silencer+stabilizer superior to stabilizer only?
But – 2 main questions in ATTR-CM
1)Which is associated with better outcomes – silencer or stabilizer?
2)Is the combination of silencer+stabilizer superior to stabilizer only?
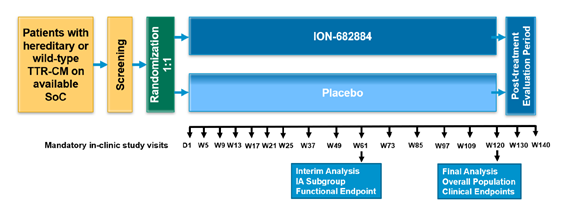
CARDIO-TTRansform (n=750 pts) is designed to answer these questions (besides efficacy of a new silencer). Primary outcome in CV mortality+CV events.
The trial is stratified by:
tafamidis use, NYHA class I/II vs III, wtATTR vs hATTR, 6MWT (< or > 350 m)
The trial is stratified by:
tafamidis use, NYHA class I/II vs III, wtATTR vs hATTR, 6MWT (< or > 350 m)
So patients are allowed to continue on tafamidis (without restrictions) and are randomized to placebo vs silencer - a key difference that separates CARDIO-TTRansform from other trials discussed below
>95% of our ATTR-CM pts are on tafamidis or AG10
>95% of our ATTR-CM pts are on tafamidis or AG10
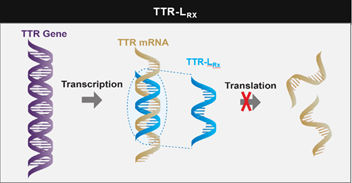
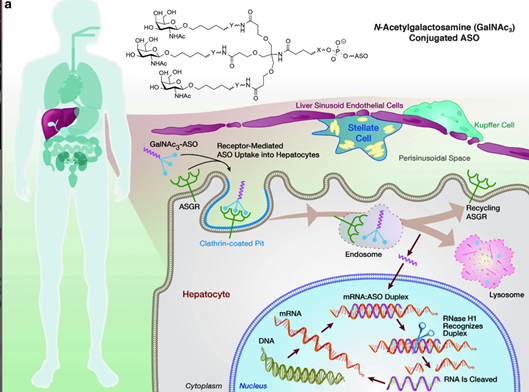
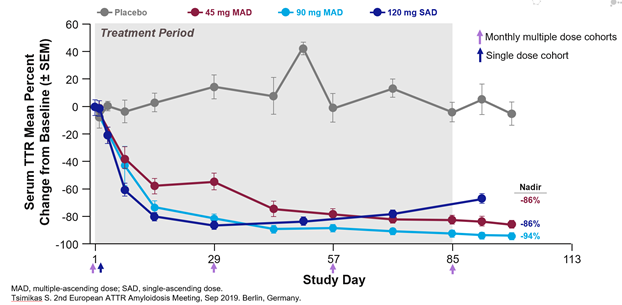
What does this silencer do? It is a 2nd generation of the already approved inotersen. Its current name is AKCEA-TTR-Lrx. Similar to inotersen, it is an anti-sense oligonucleotide (ASO) --> targets TTR pre-mRNA to inhibit TTR production (both wt and vTTR)
TTR-Lrx --> liver using ligand-conjugated ASO (LICA). Using GalNAc, it targets hepatocytes (ASGPR). Conjugation --> 30x increase in potency compared w/ unconjugated ASO--> benefit of lower doing frequency, increased potency, & dosing at lower volume.
In a phase I study, TTR-Lrx successfully silenced TTR production without adverse effects on platelets, kidney or liver.
key inclusion criteria in CARDIO-TTRansform:
ATTR-CM, NYHA class I-III, NTproBNP >/= 600 (or 1200 in AF), GFR >/= 30
ATTR-CM, NYHA class I-III, NTproBNP >/= 600 (or 1200 in AF), GFR >/= 30
Other trials in the ATTR space:
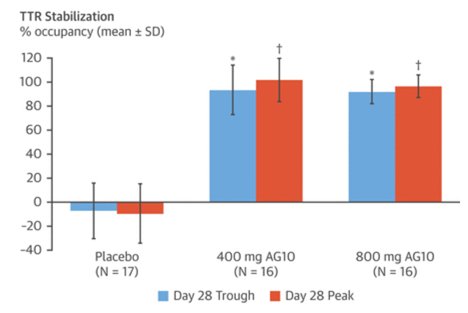
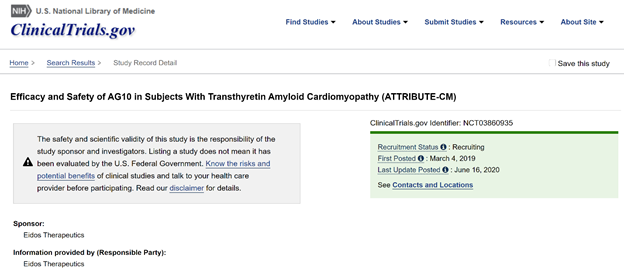
ATTRIBUTE-CM is another exciting trial of AG10, a stabilizer of TTR tetramer. N=510 (2:1), primary outcomes: 1)change in 6MWT and 2)all-cause death + CV hosp frequency. Tafamidis only allowed after 12 months
https://clinicaltrials.gov/ct2/show/NCT03860935
ATTRIBUTE-CM is another exciting trial of AG10, a stabilizer of TTR tetramer. N=510 (2:1), primary outcomes: 1)change in 6MWT and 2)all-cause death + CV hosp frequency. Tafamidis only allowed after 12 months
https://clinicaltrials.gov/ct2/show/NCT03860935
APOLLO-B is another trial in ATTR-CM testing patisiran vs placebo. N=300. Primary outcome is change from baseline at month 12 in 6MWT. A key inclusion criteria is “tafamidis naïve or on tafamidis for ≥6 months with evidence of disease progression”
https://clinicaltrials.gov/ct2/show/NCT03997383
https://clinicaltrials.gov/ct2/show/NCT03997383
HELIOS-B is another trial in ATTR-CM testing vutrisiran (q3m) against placebo in a 600 patient trial. Outcome: all-cause mortality and CV hosp. A key point in the trial design: up to 30% only can receive tafamidis
https://clinicaltrials.gov/ct2/show/NCT04153149
https://www.onlinejacc.org/content/accj/75/11_Supplement_1/3579.full.pdf
https://clinicaltrials.gov/ct2/show/NCT04153149
https://www.onlinejacc.org/content/accj/75/11_Supplement_1/3579.full.pdf
Other actively enrolling trial in the ATTR space:
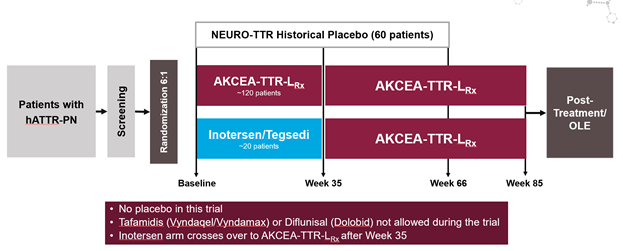
NEURO-TTRansform – testing TTR-Lrx against Inotersen. No placebo, no tafamidis. hATTR with polyneuropathy (since both are active treatment arms, no other TTR therapies allowed)
https://clinicaltrials.gov/ct2/show/NCT04136184
/end
NEURO-TTRansform – testing TTR-Lrx against Inotersen. No placebo, no tafamidis. hATTR with polyneuropathy (since both are active treatment arms, no other TTR therapies allowed)
https://clinicaltrials.gov/ct2/show/NCT04136184
/end

 Read on Twitter
Read on Twitter