I'm finding the whole conversations about covid-19 vaccines has a continued air of unreality about it. People keep saying vaccines will take a year to 18 months.
These are not normal times. Regulators are already using emergency legislation. Sometimes with not great results. h/t @FDA
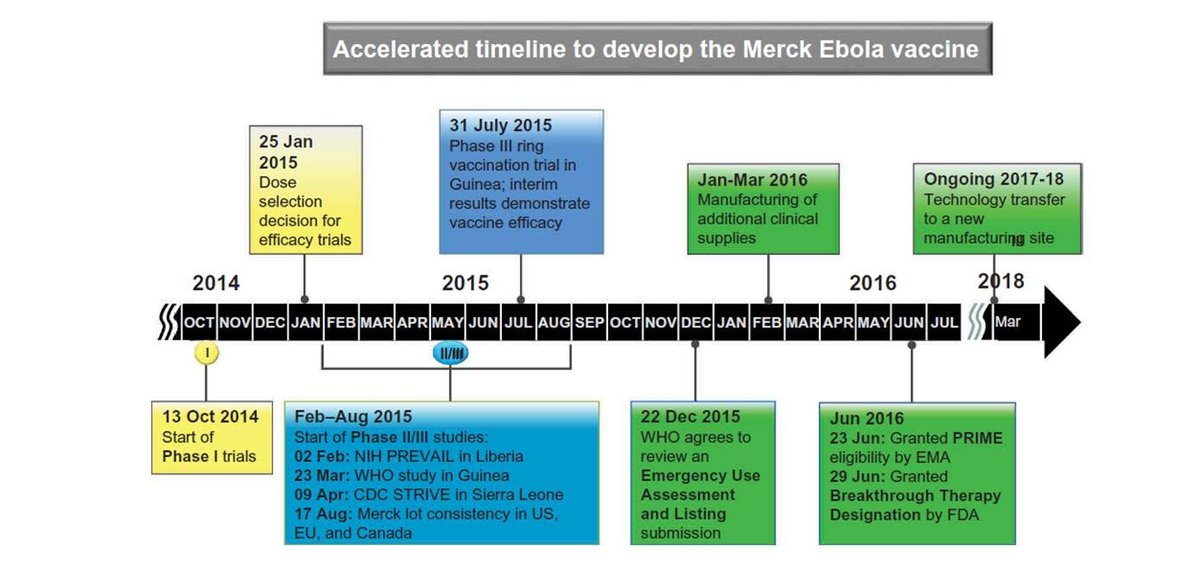
The most useful starting point for the convo is the accelerated Ebola vaccine timeline when all the nuts and bolts of testing were done in *nine months*. Trials started Oct 2014, and by July 2015 we had evidence of efficacy!..
Then there regulatory and manufacturing delays that are not relevant here. We are already manufacturing covid vaccine, and the regulatory delay in Ebola was because it didn't matter then -- the outbreak was over.
So my bottom line is that we need to think now about what happens in September if we get a positive readout on efficacy from a phase 3 covid vaccine trial.
Because we already have a lot of vaccine. The politicians will say "we have a vaccine".
People will say "give it to us!"
Because we already have a lot of vaccine. The politicians will say "we have a vaccine".
People will say "give it to us!"
We need to not be surprised by this. Now my working assumption is that there is no way a regulator could approve, even on an emergency basis, that hundreds of millions of doses of vaccine could be deployed on the basis of trials in tens of thousands of patients...
But is this correct? And if so, how do we do this properly? And, shouldn't we be sending signals now that we are not going to just roll out the Moderna and Oxford vaccine widely with one positive p3? Or am I missing something? @peterbachmd @ScottGottliebMD

 Read on Twitter
Read on Twitter