Another agnostic approval.
#Pembrolizumab
 #TMB
#TMB
 TMB
TMB cutoff >=
cutoff >=

Though I’m overall very supportive of agnostic approvals overall, not sure if TMB is the same across tissue types.
is the same across tissue types.
@US_FDA #OncoAlert #PrecisionMedicine
https://seekingalpha.com/news/3583617-fda-oks-mercks-keytruda-for-second-application-based-on-biomarker?source=tweet https://seekingalpha.com/news/3583617-fda-oks-mercks-keytruda-for-second-application-based-on-biomarker
#Pembrolizumab
 #TMB
#TMB
 TMB
TMB cutoff >=
cutoff >=

Though I’m overall very supportive of agnostic approvals overall, not sure if TMB
 is the same across tissue types.
is the same across tissue types. @US_FDA #OncoAlert #PrecisionMedicine
https://seekingalpha.com/news/3583617-fda-oks-mercks-keytruda-for-second-application-based-on-biomarker?source=tweet https://seekingalpha.com/news/3583617-fda-oks-mercks-keytruda-for-second-application-based-on-biomarker
TMB is part of the story. Cutoff of
is part of the story. Cutoff of 
 not sure if that even is applicable to a lot of GI tumors including MSI
not sure if that even is applicable to a lot of GI tumors including MSI . Even within MSI
. Even within MSI seems like the cutoff appears to be a lot higher in responders
seems like the cutoff appears to be a lot higher in responders non-responders. The 10-18ish in the intermediate TMB
non-responders. The 10-18ish in the intermediate TMB  benefit at least in GI.
benefit at least in GI.
 is part of the story. Cutoff of
is part of the story. Cutoff of 
 not sure if that even is applicable to a lot of GI tumors including MSI
not sure if that even is applicable to a lot of GI tumors including MSI . Even within MSI
. Even within MSI seems like the cutoff appears to be a lot higher in responders
seems like the cutoff appears to be a lot higher in responders non-responders. The 10-18ish in the intermediate TMB
non-responders. The 10-18ish in the intermediate TMB  benefit at least in GI.
benefit at least in GI.
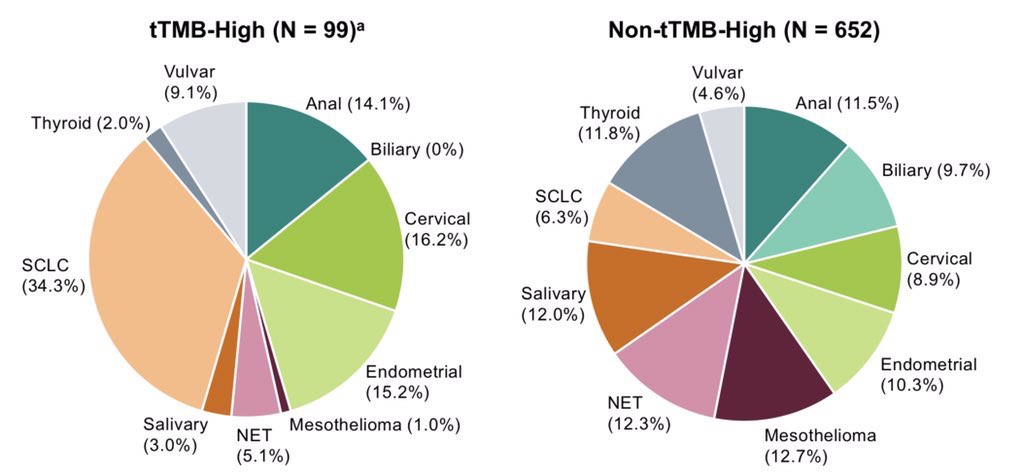
Even the patients accrued don’t meet definition of agnostic:
“158 trial accrued patients with anal, biliary, cervical, endometrial, salivary, thyroid, or vulvar carcinoma, mesothelioma, a neuroendocrine tumor, or small cell lung cancer.”
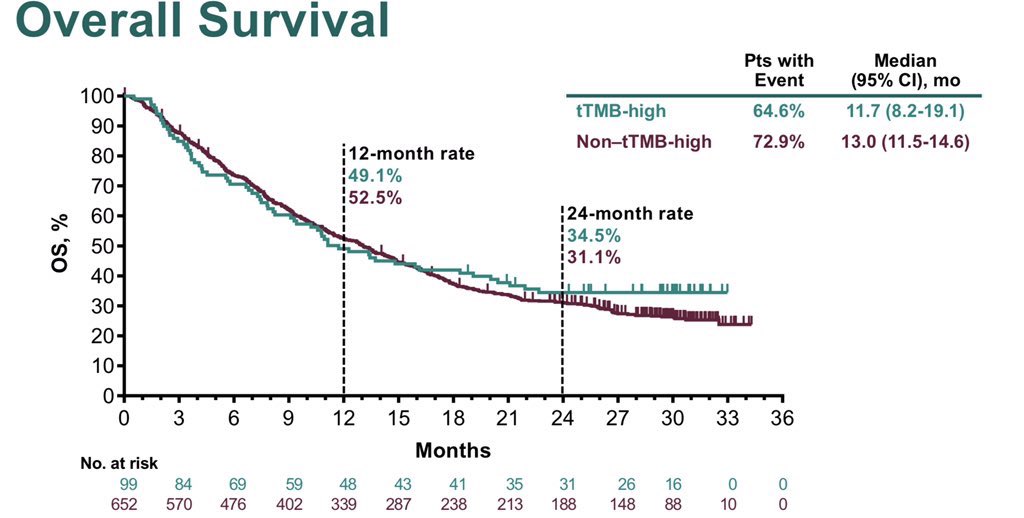
 Also baffling: OS worse in TMB
Also baffling: OS worse in TMB .
.
“158 trial accrued patients with anal, biliary, cervical, endometrial, salivary, thyroid, or vulvar carcinoma, mesothelioma, a neuroendocrine tumor, or small cell lung cancer.”
 Also baffling: OS worse in TMB
Also baffling: OS worse in TMB .
.
More on spectrum of #cancers in #Keynote158 & the tale of crossing curves similar to most #Immunotherapy studies.  tail of prolonged benefit noted in a subset of patients. As noted
tail of prolonged benefit noted in a subset of patients. As noted 
 concern is “agnostic” approval is missing:
concern is “agnostic” approval is missing:
 breast
breast
 colorectal
colorectal
 NSCLC
NSCLC
 Prostate
Prostate
@GerkIvan
 tail of prolonged benefit noted in a subset of patients. As noted
tail of prolonged benefit noted in a subset of patients. As noted 
 concern is “agnostic” approval is missing:
concern is “agnostic” approval is missing: breast
breast colorectal
colorectal NSCLC
NSCLC Prostate
Prostate@GerkIvan

 Read on Twitter
Read on Twitter