1/11). Specialty pharma detour
Oyster Point Pharma ( $OYST ) filed for IPO today.
$85M for nasal spray to treat dry eye
Nothing written on $OYST on Biotwitter yet. I have dealt with evaporative dry eye and followed this private co. Overall, skeptical about commercial prospects
Oyster Point Pharma ( $OYST ) filed for IPO today.
$85M for nasal spray to treat dry eye
Nothing written on $OYST on Biotwitter yet. I have dealt with evaporative dry eye and followed this private co. Overall, skeptical about commercial prospects
2/11). Here's the S-1
https://www.sec.gov/Archives/edgar/data/1720725/000119312519262771/d637051ds1.htm
Bookrunners: JP Morgan, Piper, Cowen
Private Investors: NEA, Versant, Vida, KKR
https://www.sec.gov/Archives/edgar/data/1720725/000119312519262771/d637051ds1.htm
Bookrunners: JP Morgan, Piper, Cowen
Private Investors: NEA, Versant, Vida, KKR
3/11). It goes without saying that this is a big market. Dry eye Rx drops Restasis ( $AGN / $ABBV) & Xiidra ( $NVS via $TAK ) sold combined $1.6B in 2018. The problem is that they treat "signs" not symptoms. And sting eyes / take months to start working https://xconomy.com/new-york/2019/02/26/oyster-point-gets-93m-to-clear-up-dry-eye-with-a-nasal-spray/
4/11). The lead Oyster drug (OC-01) works by targeting nicotinic acetylcholine receptors (nAChRs) to increase production of tears. Currently in Ph 3.
In Ph 2, patients saw stat. sig. improvement compared to placebo, in symptoms of dry eye and in Schirmer test for tear production
In Ph 2, patients saw stat. sig. improvement compared to placebo, in symptoms of dry eye and in Schirmer test for tear production
5/11). My concern w OC-01 are the AE's. The Ph 2 data seems to show the swapping of one annoying problem (dry eye) for another (post nasal drip, sneezing, throat irritation). Speaking as a non-physician, this sounds miserable. A recipe for chronic sinus infections
6/11). The company is characterizing these AE's "mild to moderate" and says that it has "no clinical safety data on patients treated with OC-01 for longer than 84 days." The drug has to be taken twice daily.
7/11). There is an effective $JNJ in-patient medical device for evaporative dry eye patients called LipiFlow. It clears obstructed meibomian glands in the eyelid with gentle heat and pressure in 10 mins. Device has been amassing efficacy data for 10 years
https://tearscience.com/lipiflow/
https://tearscience.com/lipiflow/
8/11). LipiFlow looks like the eye torture scene in "A Clockwork Orange" but it relieved a lot of my symptoms. Never tried Xiidra or Restasis. Ophthalmologist says it has really started to gain traction with the new $JNJ marketing push (acquired in 2017) https://eyewire.news/articles/johnson-johnson-vision-completes-acquisition-of-tearscience/
9/11). LipiFlow is quite expensive at $1000 out of pocket (not covered by insurance), but an economic case could be made over the long term. The treatment's relief can last a few years. It has no side effects that would require more doctor visits. And no monthly co-pays for drops
10/11). Between the stiff LipiFlow competition and the respiratory side effects, I'm skeptical about the commercial prospects for $OYST's new nasal spray for dry eye.
11/11). I also noticed that $OYST does not have a Chief Medical Officer. If the drug has so much potential then when doesn't the company have an ophthalmologist CMO on board? https://oysterpointrx.com/who-we-are/
Update October 29
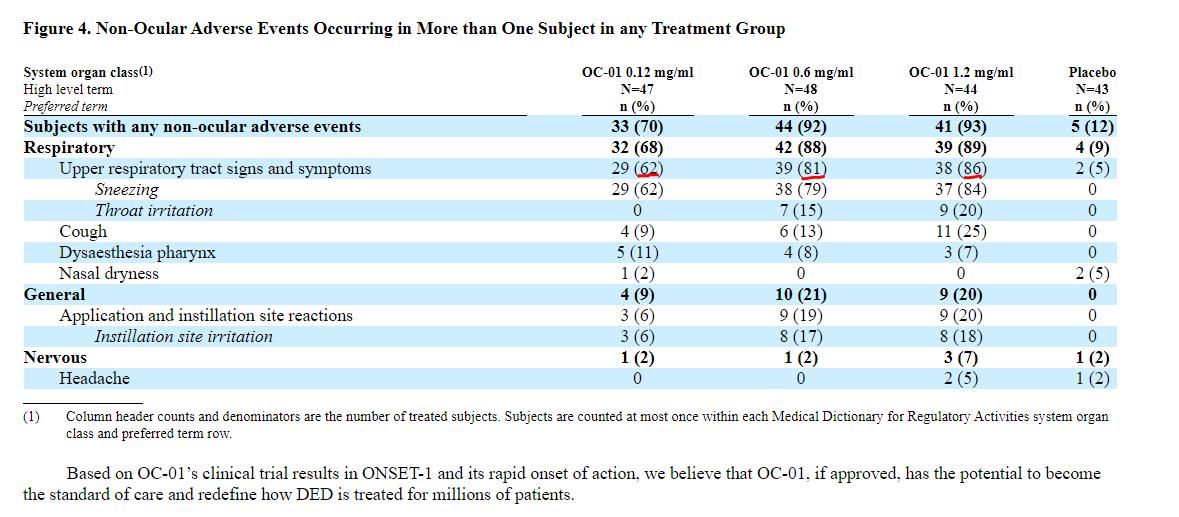
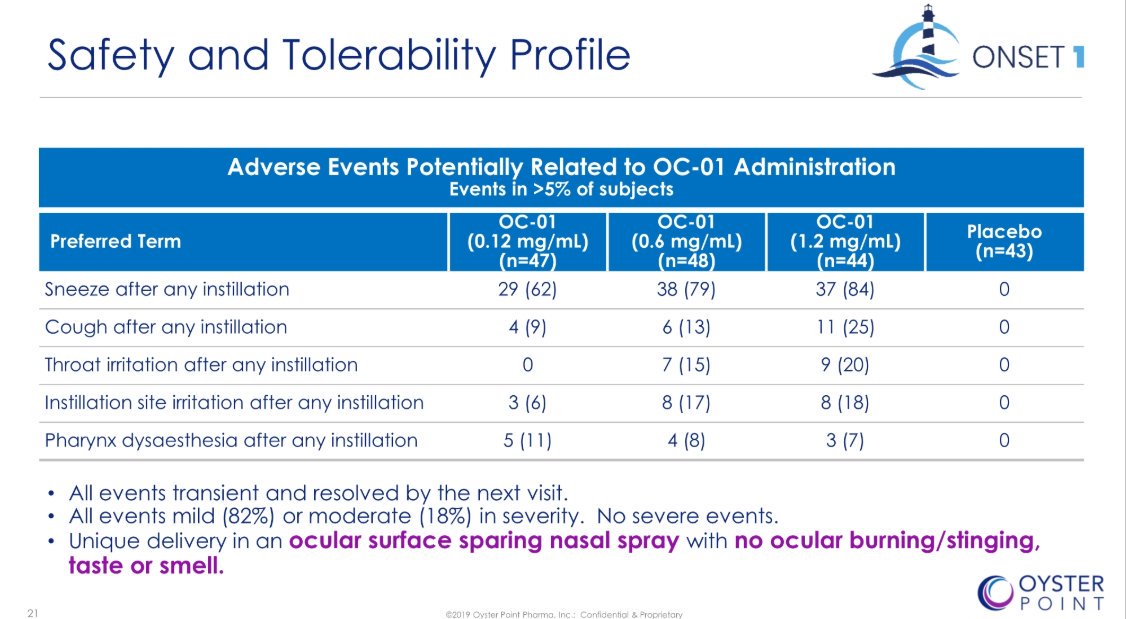
1 of 7). I am coming around to the non-ocular AE's for $OYST's drug. The company omitted this CRUCIAL slide from its S-1.
Makes it clear that most of the sneezing/coughing occurred RIGHT AFTER patients instilled the spray in their noses. Soon faded
1 of 7). I am coming around to the non-ocular AE's for $OYST's drug. The company omitted this CRUCIAL slide from its S-1.
Makes it clear that most of the sneezing/coughing occurred RIGHT AFTER patients instilled the spray in their noses. Soon faded
Update October 29
2 of 7). $OYST mgmt. comments in this vid (15:06)
•Pts. often sneeze because spray is close to trigeminal nerve
•Temporally related to admin: 10-20 secs after spray
•Numbers in slide represent pts. that sneezed ANY TIME during trial
2 of 7). $OYST mgmt. comments in this vid (15:06)
•Pts. often sneeze because spray is close to trigeminal nerve
•Temporally related to admin: 10-20 secs after spray
•Numbers in slide represent pts. that sneezed ANY TIME during trial
Update October 29
3 of 7). More $OYST mgmt. comments in vid
•Drug meant to be delivered to ANTERIOR nasal cavity, NOT DEEP inside cavity like ALLERGY NASAL SPRAYS
•Spraying drug deep causes throat irritation
•When pts. taught not to do this, CEO says: "we have no problems"
3 of 7). More $OYST mgmt. comments in vid
•Drug meant to be delivered to ANTERIOR nasal cavity, NOT DEEP inside cavity like ALLERGY NASAL SPRAYS
•Spraying drug deep causes throat irritation
•When pts. taught not to do this, CEO says: "we have no problems"
Update October 29
4 of 7). More $OYST mgmt. comments
•It's a 50 microliter spray
•Most allergy sprays are 150 microliters
•One-third the size of allergy sprays
4 of 7). More $OYST mgmt. comments
•It's a 50 microliter spray
•Most allergy sprays are 150 microliters
•One-third the size of allergy sprays
Update October 29
5 of 7). $OYST drug's benefits (fast-acting tear production that lasts throughout day with no need for drops) seem to be worth the non-ocular AE's. Slide & video clears question up.
Could be complementary to $JNJ LipiFlow & Lotemax https://www.reviewofcontactlenses.com/article/topical-steroids-and-the-treatment-of-dry-eye
5 of 7). $OYST drug's benefits (fast-acting tear production that lasts throughout day with no need for drops) seem to be worth the non-ocular AE's. Slide & video clears question up.
Could be complementary to $JNJ LipiFlow & Lotemax https://www.reviewofcontactlenses.com/article/topical-steroids-and-the-treatment-of-dry-eye
Update October 29
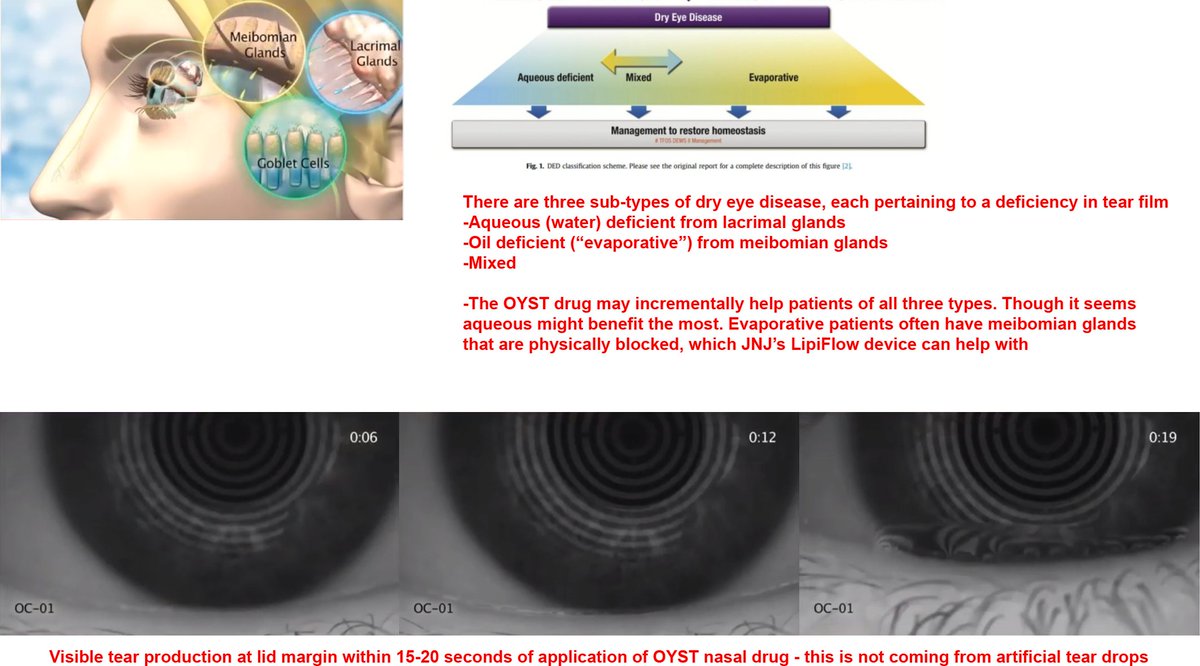
6 of 7). Here is the $OYST drug in action. The tear production that sets in within 15-20 seconds of application is sure to provoke a visceral reaction from any dry eye patients. Looks nice.
This was in the video above at 6:40 mark
6 of 7). Here is the $OYST drug in action. The tear production that sets in within 15-20 seconds of application is sure to provoke a visceral reaction from any dry eye patients. Looks nice.
This was in the video above at 6:40 mark
Update October 29
7 of 7). There is one MAJOR safety question left mostly unanswered in the $OYST S-1. It does not pertain to the respiratory AE chart. I will perhaps examine it tomorrow in more detail here
7 of 7). There is one MAJOR safety question left mostly unanswered in the $OYST S-1. It does not pertain to the respiratory AE chart. I will perhaps examine it tomorrow in more detail here
UPDATE: There is still one neuro/psych safety question left unanswered.
But $OYST did reveal positive efficacy results in Ph2 MYSTIC at #JPM2020
Improved Schirmer score vs placebo in Ph2
$ALDX $KALA $AERI $AUPH $OCUL
#DryEye https://twitter.com/syinvesting/status/1216624366168858624
But $OYST did reveal positive efficacy results in Ph2 MYSTIC at #JPM2020
Improved Schirmer score vs placebo in Ph2
$ALDX $KALA $AERI $AUPH $OCUL
#DryEye https://twitter.com/syinvesting/status/1216624366168858624
Another user comment https://twitter.com/levi_ofir/status/1216646320351477760
$OYST topline Ph3 data for dry eye disease (DED)
Conf call at 8 am EST today
In the 30 day trial, most common AE was sneeze
Occurred with 50% of administrations. Was transient and mild, occurring right after the spray, as seen in Ph2
@NewportBchTweet
https://investors.oysterpointrx.com/node/6886/pdf
Conf call at 8 am EST today
In the 30 day trial, most common AE was sneeze
Occurred with 50% of administrations. Was transient and mild, occurring right after the spray, as seen in Ph2
@NewportBchTweet
https://investors.oysterpointrx.com/node/6886/pdf

 Read on Twitter
Read on Twitter