1. Thanks everyone for engaging!!! 

Differential diagnosis includes and is not limited to:
Benign lesions/tumors
- Dermal scar
- Neurofibroma
- Blue nevus
Malignant tumors
- Spindle cell carcinoma
- AFX
- DFSP
- Desmoplastic leiomyosarcoma
- MPNST


Differential diagnosis includes and is not limited to:
Benign lesions/tumors
- Dermal scar
- Neurofibroma
- Blue nevus
Malignant tumors
- Spindle cell carcinoma
- AFX
- DFSP
- Desmoplastic leiomyosarcoma
- MPNST
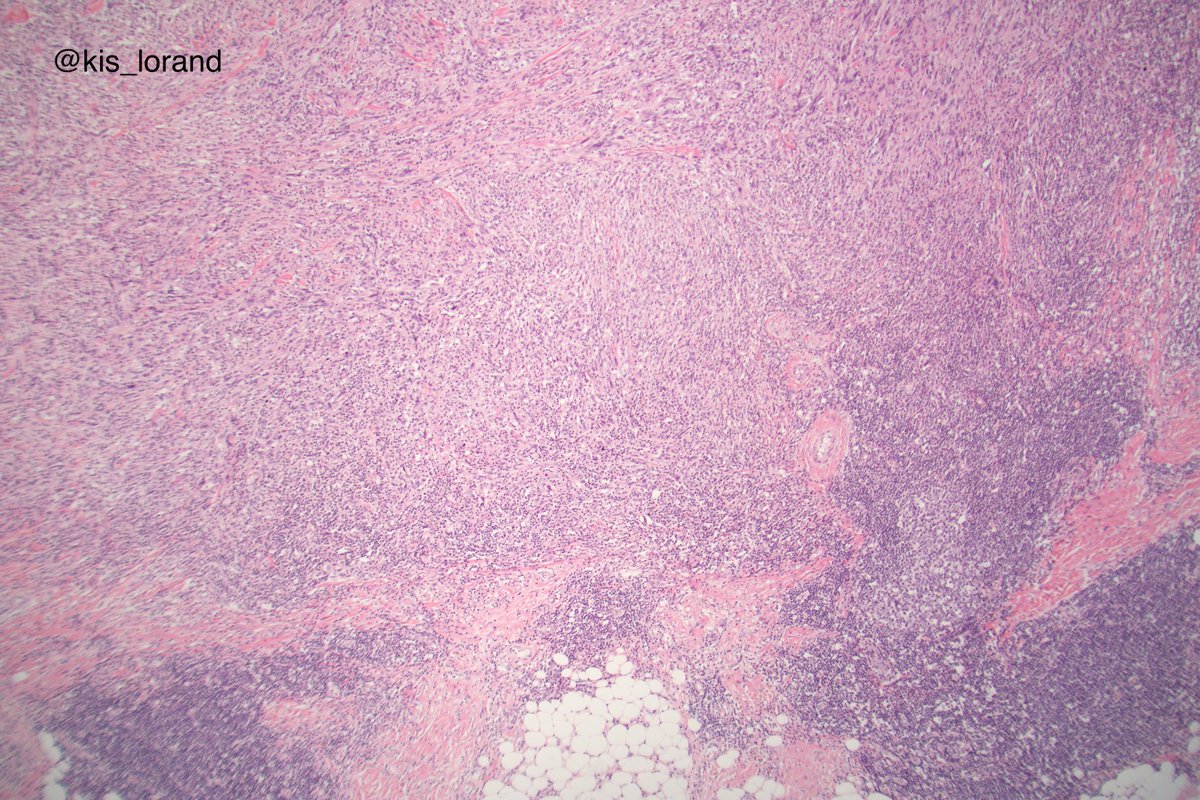
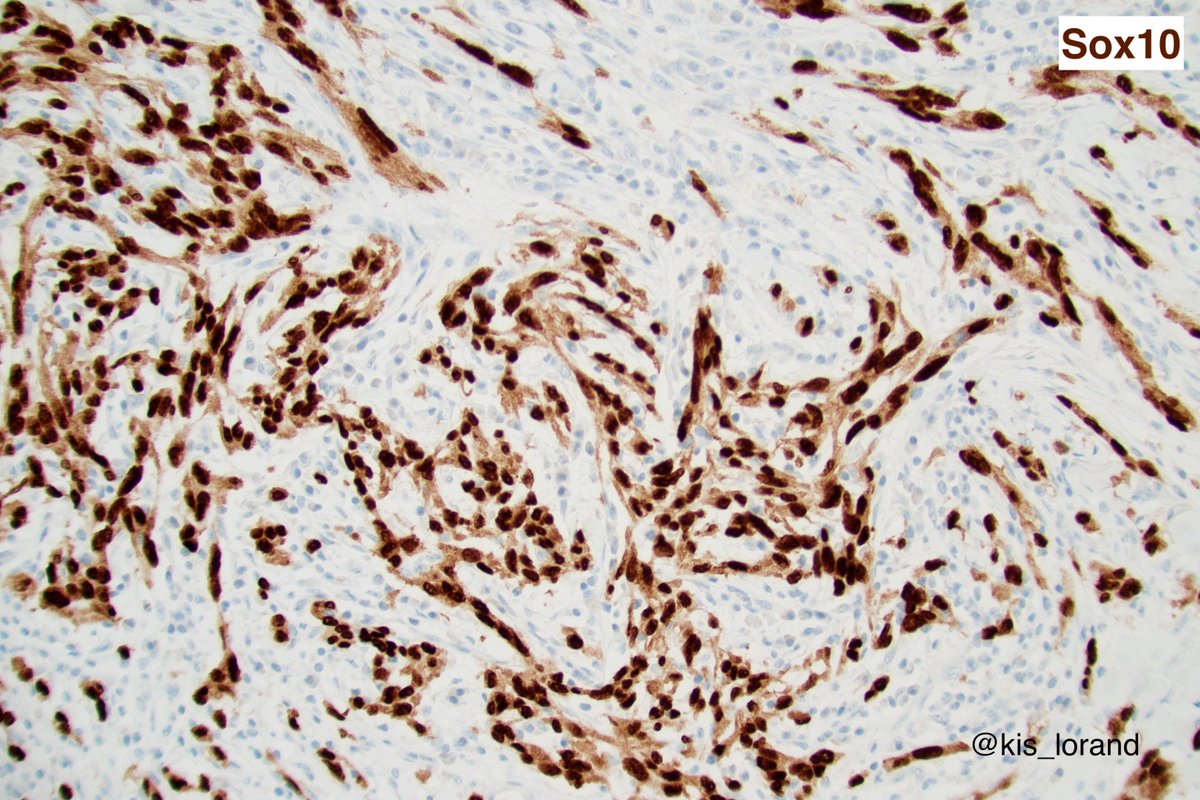
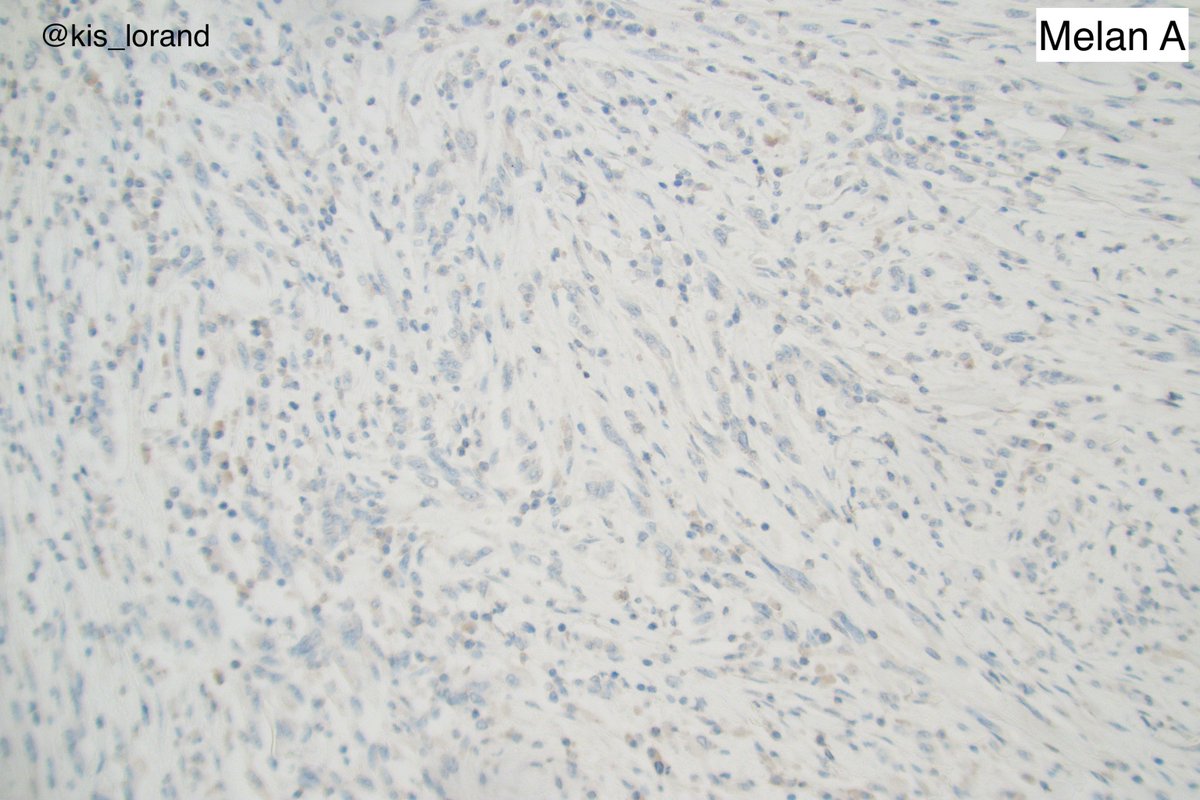
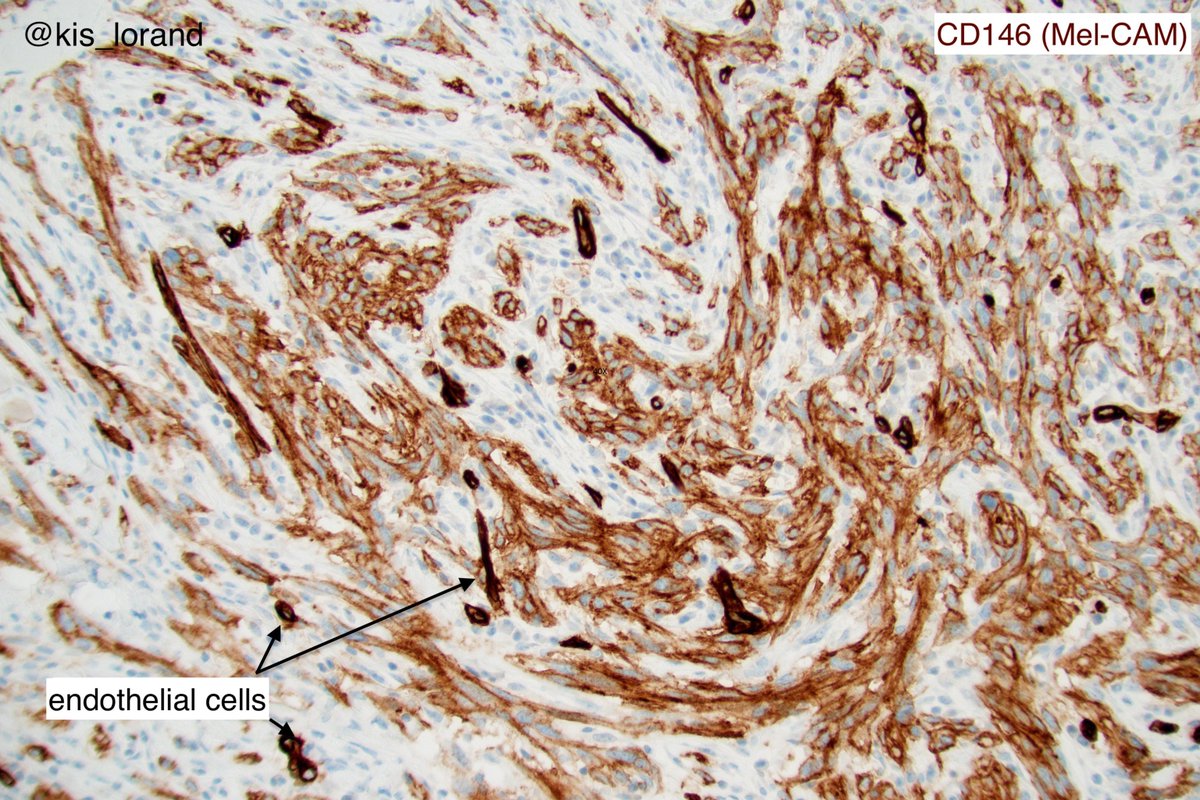
2. The pictures show an intradermal, well circumscribed cellular lesion without a junctional component, surrounded by lymphoid aggregates. The high power pictures shows clear cut atypia. Mitoses were present but not shown in the pictures. The IHCs favour a melanocytic tumor (
3. (the homogenous S100 and Sox10 also favours melanoma over MPNST that show focal, week labeling for these markers).
So the diagnosis is an invasive melanoma, spindle cell or desmoplastic probably of less importance from the point of view of the patients management. Even so...
So the diagnosis is an invasive melanoma, spindle cell or desmoplastic probably of less importance from the point of view of the patients management. Even so...
4. the tumor is too cellular for a desmoplastic melanoma (DM); DM is a pink lesion on low power, looks like a scar; the neoplastic cells are infiltrating a dense fibrous stroma.
DM is a better defined entity while spindle cell melanoma is a more heterogeneous entity.
DM is a better defined entity while spindle cell melanoma is a more heterogeneous entity.
5. Interestingly the DMs are rarely associated with lymph node metastasis but have a higher rate of local recurrence (compared to the conventional melanomas). I cannot explain why but this is exactly what I would expect from such spindle cells, they cannot crawl into the vessels?
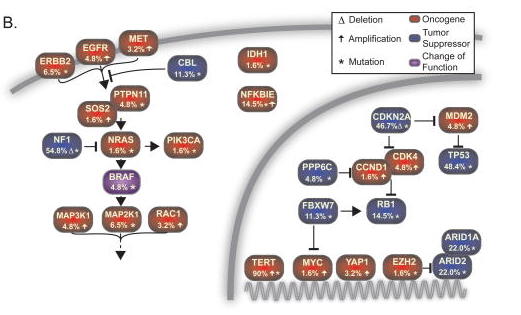
6. Molecular: exome sequencing of DM showed high mutational burden, mutations in the promoter of NFKBIE gene, no BRAF V600 mutations but instead in about 55% of the cases inactivation of the NF1 tumor suppressor.
The article is freely available in PMC https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4589486/
The article is freely available in PMC https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4589486/
7. Another interesting observation is that DM belongs to the very few cancer subtypes that showed objective tumour responses to anti-PD1/anti-PD-L1 immunotherapy in about 70% of the cases.
Freely available in PMC https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5773412/
Freely available in PMC https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5773412/
8. Let's keep the thread open with the following question. What other cancer subtypes showed objective responses to PD-1/PD-L1 immunotherapy (monotherapy) in the range of 50% or above?
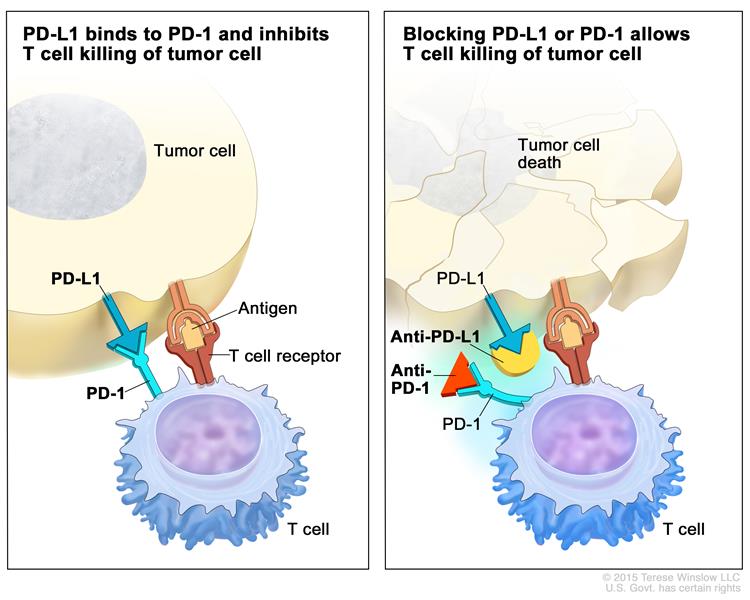
9. Before we look at the results let us consider the PD-1/PD-L1 immunotherapy in a nutshell
The simplified picture is that the cancer cells or the tumor-associated immune cells express PD-L1 that interacts with the PD-1 receptor on the CD8+ T cells and results in the
The simplified picture is that the cancer cells or the tumor-associated immune cells express PD-L1 that interacts with the PD-1 receptor on the CD8+ T cells and results in the
10. inhibition/deactivation of the cytotoxic T cells.
Breaking up this PD-L1-PD1 interaction with therapeutic blocking antibodies results in the reinvigoration/reactivation of the CD8+ T cells and tumor killing. Nice, this is what we want

Breaking up this PD-L1-PD1 interaction with therapeutic blocking antibodies results in the reinvigoration/reactivation of the CD8+ T cells and tumor killing. Nice, this is what we want


11. Check out the ”immune checkpoint inhibitor” term at the
NCI Dictionary of Cancer Terms
https://www.cancer.gov/publications/dictionaries/cancer-terms/def/immune-checkpoint-inhibitor
NCI Dictionary of Cancer Terms
https://www.cancer.gov/publications/dictionaries/cancer-terms/def/immune-checkpoint-inhibitor
12. Knowing that these novel therapies (so called ”PD-1/PD-L1 checkpoint inhibitors”) are expensive, have autoimmune side effects, and only a minority if the patients will benefit from the therapy the question is how can we identify which patient will benefit from the therapy.
13. There are several therapeutic antibodies available for such immunotherapy; some are targeting the PD-1 receptor (pembrolizumab, nivolumab, cemiplimab), while others the ligand called PD-L1 (atezolizumab, durvalumab, avelumab).
14. In certain cancer subtypes (melanoma,kidney cancer, classic Hodgk lymphoma) there are no clinically validated biomarkers that can help us in predict which patient will respond to the therapy; this is why in these cancer subtypes all patients are candidate for these therapies
15. In clinical praxis there are only 2 validated predictive biomarkers for these treatments:
a). PD-L1 protein expression by IHC in tumor cells or tumor-infiltrating immune cells (useful to a certain degree to predict responses in NSCLC, gastric and gastro-esophageal
a). PD-L1 protein expression by IHC in tumor cells or tumor-infiltrating immune cells (useful to a certain degree to predict responses in NSCLC, gastric and gastro-esophageal
16. adenocarcinoma, cervical, urothelial, head and neck cancers)
b). microsatellite instability-high (MSI-H) or mismatch repair deficiency (dMMR) in any cancer subtype
b). microsatellite instability-high (MSI-H) or mismatch repair deficiency (dMMR) in any cancer subtype
17. So what is the answer to the original question? 
 The thread became too long, but I hope you learned something new by reading it...
The thread became too long, but I hope you learned something new by reading it...

 The thread became too long, but I hope you learned something new by reading it...
The thread became too long, but I hope you learned something new by reading it...
18. In the KEYNOTE-087 single-arm phase II study treatment with pembrolizumab (a PD-1 blocking antibody) in relapsed or refractory classic Hodgkin lymphoma patients the ORR was 69% (complete response rate 24%). http://ascopubs.org/doi/abs/10.1200/JCO.2016.72.1316
19. In a preplanned interim analysis of a phase 2 study in 39 patients with metastatic Merkel cell carcinoma, avelumab (anti- PD-L1 antibody) treatment resulted in a confirmed ORR in 62.1% of the patients. https://jamanetwork.com/journals/jamaoncology/fullarticle/2675910
20. In the KEYNOTE-024 open-label, phase 3 trial in previously untreated advanced NSCLC with PD-L1 expression on at least 50% of tumor cells the response rate in the pembrolizumab group was 44.8% compared to chemotherapy 27.8 %. https://www.nejm.org/doi/full/10.1056/NEJMoa1606774
21. In the expansion cohorts of the phase 1 study, a response to cemiplimab (anti-PD-1 antibody) was observed in 50% of the patients. In the metastatic-disease cohort of the phase 2 study the response rate was 47%.
https://www.nejm.org/doi/full/10.1056/NEJMoa1805131
https://www.nejm.org/doi/full/10.1056/NEJMoa1805131

 Read on Twitter
Read on Twitter