So - I wanted to address a topic that some of you have been asking about: solid-state batteries. What are they? When are they coming? Is the hype to be believed?
Well, here comes another thread which will hopefully shed some light on this...
Well, here comes another thread which will hopefully shed some light on this...
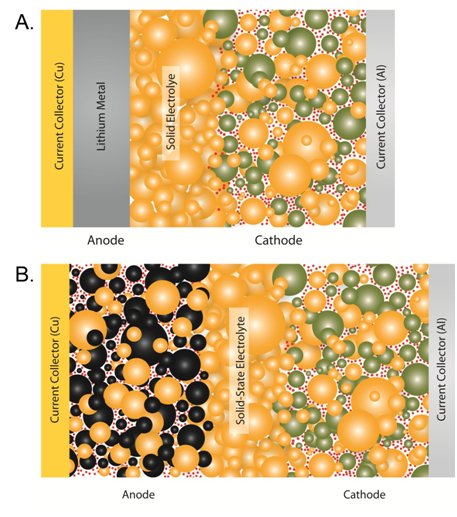
Probably the first thing to say is that they are not conceptually very different from Li-ion batteries. In a solid-state battery, the liquid electrolyte is simply replaced with a solid which does the same job, i.e., separate the electrodes and conduct Li+ (img cred: UC San Diego)
"Solid-state" has come to mean electrolytes which are inorganic solids, such as ceramic lithium-conducting materials. There are a number of different types or classes of these, but I won't get stuck into this here...
So why solid-state? An obvious advantage is safety - a ceramic will not burn if the cell is short-circuited, damaged or overcharged; and this might also allow for a wider operating temperature range.
Higher energy density is also a potential advantage as well, since an intrinsically safer cell may mean a full battery pack will have less requirements for safety systems and cooling. The use of Li metal electrodes might also be possible, increasing energy at the cell level too.
Some of the major types of solid electrolyte also have conductivities in the range of the liquids already in use, so there is not necessarily any disadvantage there. So what's the catch?!
Well, an all-solid battery needs new manufacturing methods, with new materials, and maybe these don't scale easily or as quickly as we would like.
But there are also more fundamental problems which are proving tricky to solve...
But there are also more fundamental problems which are proving tricky to solve...
In normal Li-ion cells, the liquid penetrates through the pores of the electrodes, wetting all the small particles well - this is essential, else ions can't be transported where needed. It is rather harder to get this good contact with solids! (see again the diagram further up).
Some electrolytes, for example the "sulfide" family (e.g. lithium germanium phosphorus sulfide, LGPS) are quite soft, so achieving a good contact between materials is easier. Unfortunately, these materials only tolerate a rather narrow range of cell voltages!
Without good contact, cells will have very high resistance, and good contact requires high pressure on the cells. The problem of high resistance will likely get worse with thicker electrodes (and this is probably one big reason why we see relatively few practical prototypes).
Another big issue is related to the Li metal. Solid-state batteries would have a big advantage over conventional Li-ion if they can enable the use of Li metal as the negative electrode - in principle, Li dendrites can't grow through a solid, right?
Unfortunately, most of the solid electrolytes are not stable in direct contact with lithium. One major type, LLZO (lithium lanthanum zirconium oxide) is considered stable, but it is known now that Li dendrites in fact rapidly grow through the tiniest imperfections in the solid!
So what to make of all of this?
To be honest, it is very hard to know what is coming. Many people in the industry are in the dark too - they haven't seen any prototypes, and some are skeptical that companies claiming to make solid-state batteries can do so.
To be honest, it is very hard to know what is coming. Many people in the industry are in the dark too - they haven't seen any prototypes, and some are skeptical that companies claiming to make solid-state batteries can do so.
There have also been some relatively high-profile controversies, such as that of Sakti3, who attracted huge investments from venture capitalists, and only ever demonstrated cells no more powerful than the small scale cells we produce in our lab.
For me - I think solid state is "not unrealistic" in principle - the science is certainly valid - but I won't get too excited about announcements of large scale production until I see verifiable prototypes. A breakthrough may well have been made for all I know!

 Read on Twitter
Read on Twitter